Figure 1.
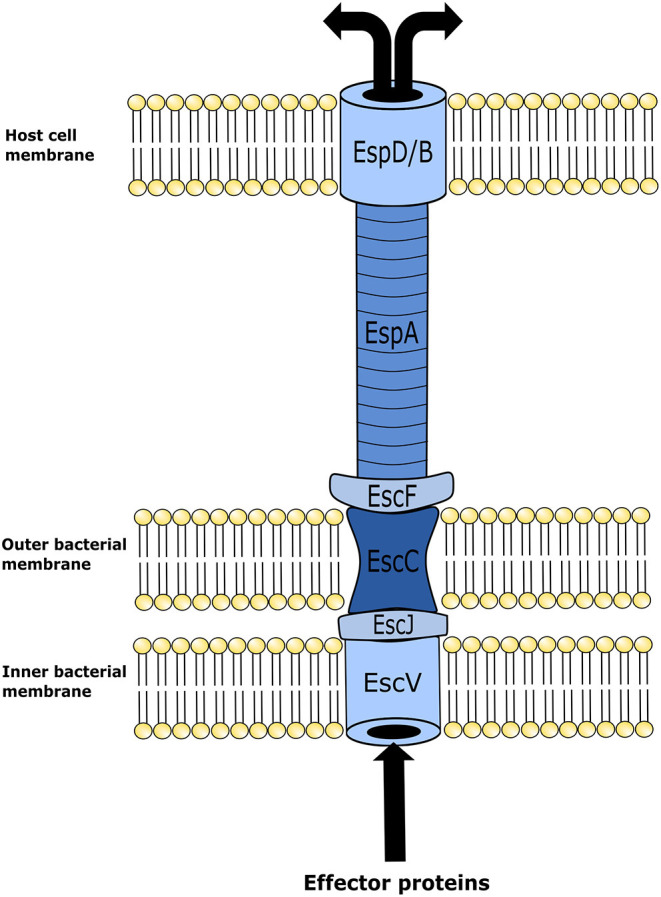
Illustration of enterohemorrhagic Escherichia coli (EHEC) type three secretion system structure. This mechanism is utilized by EHEC to introduce effector proteins into the host cell. Upon locus of enterocyte effacement (LEE) activation, the proteins EscV and EscC establish themselves in the inner and outer membranes of the bacterium, respectively, forming an annular complex. The structural lipoprotein EscJ is then placed within the periplasmic space between EscV and EscC. In conjunction, these proteins make a corridor on the bacterial membrane for effector proteins to be transported out of the cell. In turn effector proteins are moved from the bacterial membrane into the host cell using a needle-like structure that is elongated from the bacterial membrane structure to an annular complex formed in the host cell membrane. This needle-like structure is comprised of the E. coli secreted proteins, EscF and EspA. EspA forms bonds with EscF, and polymerizes into the hollow needle-like structure that extends out and allows the connection with the host cells of the intestinal epithelium. In combination with EspA, protein EspB and EspD form a pore on the cell membrane of the host epithelial cell. Effector proteins then travel through the fully formed secretion system from the bacterium into the host cell.
