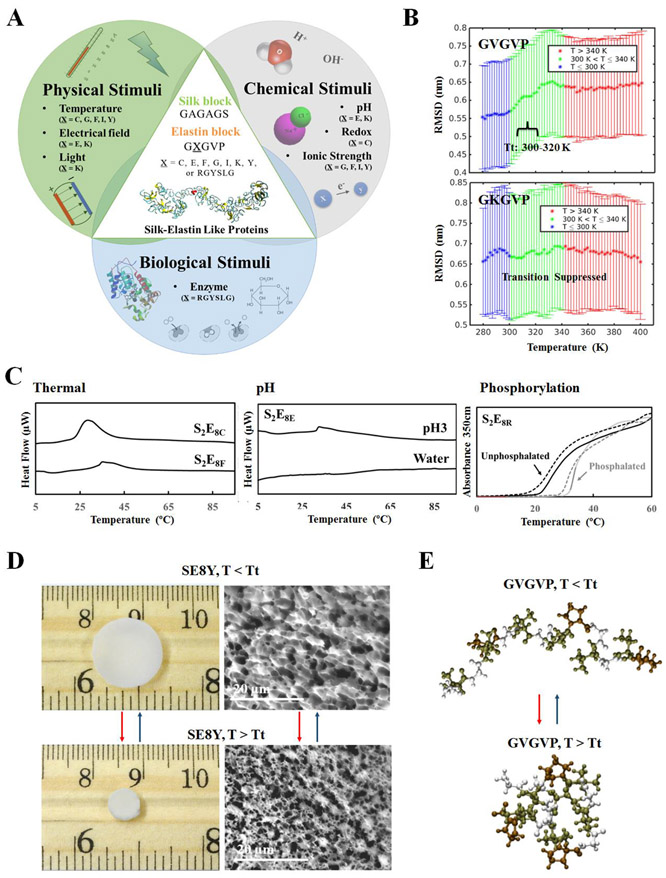
Figure 5.
Integrated modeling and experimental approach for the design of stimuli-responsive biomaterials. (A) Schematic of the sequence dependent stimuli-responsive features of SELPs. By varying position “X” amino acid in the elastin domains, SELPs can be designed to predictably respond to physical stimuli (e.g., temperature, electric field, light), chemical stimuli (e.g. pH, redox state, ionic strength), and biological stimuli (e.g., enzymatic triggers). (B) Root mean square deviation (RMSD) values for the elastin domain (GVGVP)(GXGVP)(GVGVP) from REMD simulations. RMSD values for X = V indicate a transition from extended to folded conformations. RMSD values for the X = K sequence suggest a suppressed transition in the elastin domain. The analysis suggests that the inverse temperature transition is governed by the “X” residue in elastin domain. (C) Differential scanning calorimetry and turbidity profiles of SELP solution demonstrated that SELPs can be designed to respond to a variety of stimuli (e.g., temperature, pH and phosphorylation). (D) Optical and SEM images of SELP, below and above Tt, exemplified by SE8Y with sequence of [(GAGAGS)(GVGVP)4(GYGVP)(GVGVP)3]14. Result demonstrated that SELPs can be fabricate into hydrogel for stimuli-responsive actuators. (E) Snapshots of the elastin domain (GVGVP)(GXGVP)(GVGVP), from REMD simulation, below and above Tt. An extended conformation is observed below Tt and a folded configuration is present above Tt. Adapted with permission from ref. 24. Copyright 2016 WILEY–VCH.