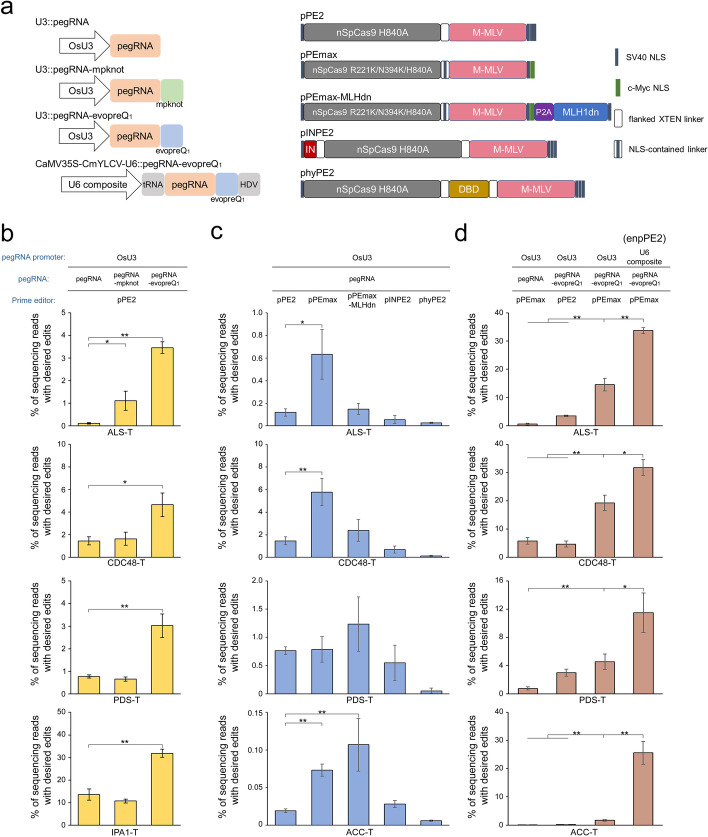
Fig. 1.
Optimization of plant prime editing systems in rice. a Schematic illustration of the engineering of plant prime editing systems. Left, pegRNA expression systems. OsU3, rice U3 promoter; U6 composite, composite promoter of the CaMV 35S enhancer, CmYLCV promoter, and shortened U6-26 promoter; tRNA (Gly-tRNA) and HDV ribozyme were built in the expression cassette driven by the U6 composite promoter to precisely process pegRNAs. Right, engineered prime editors. DBD, hRad51-ssDBD; IN, IGFpm1-NFATC2IPp1 dual peptide; P2A, porcine teschovirus-1 2A self-cleaving peptide; MLH1dn, dominant-negative mutant of the MLH1 protein; NLS, nuclear localization signal. The elements were codon-optimized for rice expression. b Editing efficiency of pPE2 in stably transformed callus cells with canonical pegRNAs (pegRNA), pegRNAs engineered by appending the structured RNA motif sequence of mpknot (pegRNA-mpknot) or evopreQ1 (pegRNA-evopreQ1) at the 3′ end with an 8-nt linker. c Editing efficiency of pPE2 variants with canonical pegRNAs in stably transformed callus cells. d Editing efficiency of pPEmax with pegRNA-evopreQ1 in stably transformed callus cells. The ratios of reads carrying the desired mutations to total clean reads were calculated as the prime editing efficiency. Data are presented as the mean value and standard deviation of three biological replicate samples from independent transformations. Statistical differences were determined by two-tailed t tests. *, p<0.05; **, p<0.01