Figure 3.
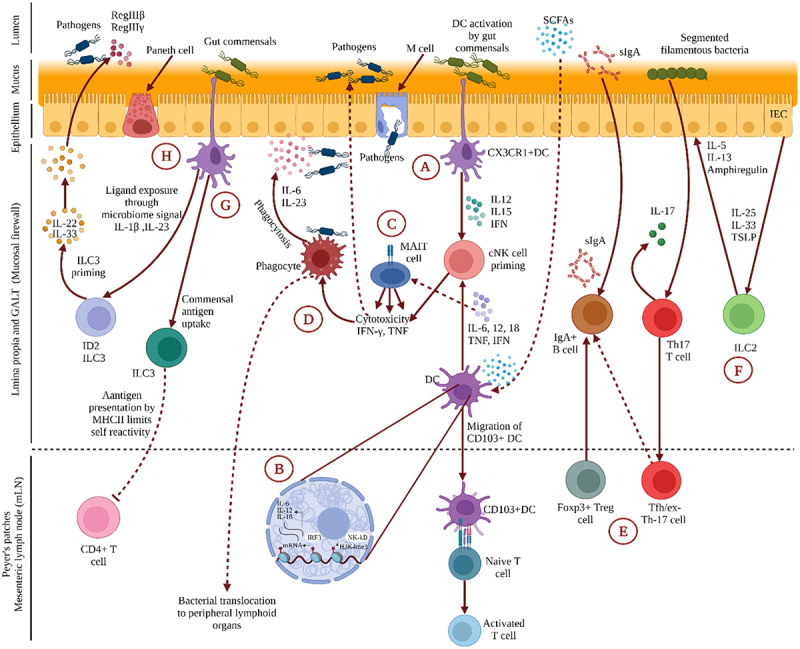
Gut-Skin communication through immuno-cross-linking. This illustration represents the immunological crosstalk between the gut and skin. (A) CX3CR1+ DCs generate dendrites for phagocytosis at homeostatic condition, whereas CD103+ DCs relocate to Peyer’s patches or mesenteric lymph nodes to deliver antigens to naive T lymphocytes. DC secretes interleukin (IL)-12, IL-15, and interferon (IFN) in response to commensal activation to stimulate conventional NK (cNK) cells. (B) As metabolic by-products, short-chain fatty acids (SCFAs) upregulate H3K4me3 in DC and enhance the production of IL-6, IL-12, IFN, and tumor necrosis factor (TNF), which is an alternative way to train cNK cells. Trained cNK cells have the necessary cytotoxicity and cytokine production capacity to fight bacteria and viruses. (C) MAIT cells can be directly stimulated to create IFN-γ by IL-12 or IL-15 in combination with IL-18 produced by APCs in response to TLR ligands. (D) TNF-like protein, a gut-associated pro-inflammatory cytokine, activates MAIT cells when coupled with IL-12 and IL-18. Phagocytes help the body defend itself by phagocytosing and producing cytokines like IL-6 and IL-23. (E) Foxp3+ Treg cells and Tfh/ex-Th17 cells cluster in Peyer’s patches, promoting B cell class switching and secretory (s)IgA production. These help to compartmentalize the commensal microbiome and modulate the diversity of the homeostatic microbiome. (F) ILC2 is activated by IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) produced by intestinal epithelial cells (IEC) in response to commensal bacteria. (G) ILC3 expressing MHC II is capable of delivering commensal antigens to CD4 + T cells, reducing their self-reactivity. (H) In an ID2-dependent manner, microbial signals are also used to prime ILC3. ILC3, which has been primed secretes IL-22 and participates in the pathogen defense by stimulating the synthesis of antimicrobial peptides, such as REGIIIβ and REGIIIγ. This illustration was based on 55–58 and created in BioRender.com (2022).
