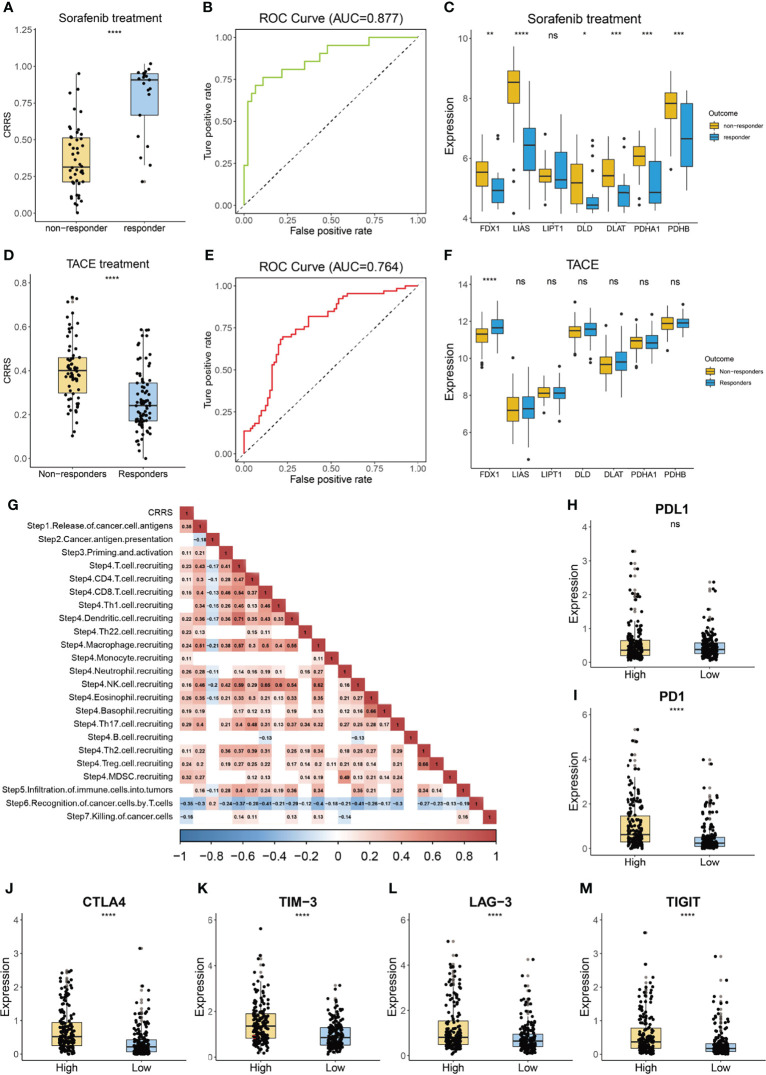
Figure 7.
Guidance of CRRS in the therapy for HCC patients. (A) The box and dot plot showing the value of CRRS between responders and non-responders to sorafenib. Wilcoxon test was used for data analyses. (B) The AUC value of CRRS in predicting the efficiency of sorafenib in HCC patients. (C) The boxplot showing the expression of cuproptosis-related genes in the responders and non-responders to sorafenib. Wilcoxon test was used for data analyses. (D) The box and dot plot showing the value of CRRS between responders and non-responders to TACE. Wilcoxon test was used for data analyses. (E) The AUC value of CRRS in predicting efficiency of TACE in HCC patients. (F) The boxplot showing the expression of cuproptosis-related genes in responders and non-responders to TACE. Wilcoxon test was used for data analyses. (G) Correlation between CRRS and immune activity scores of each step of the Cancer-Immunity Cycle. Data were analyzed using Pearson correlation analysis. (H–M) The box and dot plot showing the expression of PDL1 (H), PD-1 (I), CTLA-4 (J), TIM-3 (K), LAG-3 (L), and TIGIT (M) between HCC patients from the low- or high-CRRS subgroups. Wilcoxon test was used for data analyses. The p values were shown as *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. ns for not significant.