Abstract
Simple Summary
Vaccination represents one of the most relevant strategies to prevent and control infectious diseases in aquaculture. However, vaccines have failed to control and prevent Piscirickettsia salmonis, a bacterium that causes large economic losses to the industry. Therefore, we evaluated the performance of two commercial vaccines in Atlantic salmon through a cohabitation challenge (healthy fish were challenged by cohabitation with infected fish) of the two most prevalent and ubiquitous Piscirickettsia genetic variants in Chile. We found no evidence that vaccines confer protection against the LF-89 or EM-90 genogroups in Atlantic salmon.
Abstract
In Atlantic salmon, vaccines have failed to control and prevent Piscirickettsiosis, for reasons that remain elusive. In this study, we report the efficacy of two commercial vaccines developed with the Piscirickettsia salmonis isolates AL100005 and AL 20542 against another two genogroups which are considered highly and ubiquitously prevalent in Chile: LF-89 and EM-90. Two cohabitation trials were performed to mimic field conditions and vaccine performance: (1) post-smolt fish were challenged with a single infection of LF-89, (2) adults were coinfected with EM-90, and a low level coinfection of sea lice. In the first trial, the vaccine delayed smolt mortalities by two days; however, unvaccinated and vaccinated fish did not show significant differences in survival (unvaccinated: 60.3%, vaccinated: 56.7%; p = 0.28). In the second trial, mortality started three days later for vaccinated fish than unvaccinated fish. However, unvaccinated and vaccinated fish did not show significant differences in survival (unvaccinated: 64.6%, vaccinated: 60.2%, p = 0.58). Thus, we found no evidence that the evaluated vaccines confer effective protection against the genogroups LF-89 and EM-90 of P. salmonis with estimated relative survival proportions (RPSs) of −9% and −12%, respectively. More studies are necessary to evaluate whether pathogen heterogeneity is a key determinant of the lack of vaccine efficacy against P. salmonis.
Keywords: pentavalent vaccine, bacterin vaccine, live attenuated vaccine, monovalent vaccine, Piscirickettsiosis, Salmo salar, cohabitation, sea lice, vaccine efficacy
1. Introduction
Piscirickettsia salmonis is a major concern for the Chilean salmon industry, causing economic losses of USD 700 million per year [1,2]. Piscirickettsiosis is an exceptionally contagious disease, with mortalities of over 50% in regions of Chile where prevalence is high [3]. While Chile, the second-largest global producer of salmon, is by far the country most affected by this disease, it also affects the other main salmon producing countries, namely Norway, Canada, and Scotland [4,5,6,7].
Vaccination has been widely used as a control strategy to prevent Piscirickettsiosis [8], but unfortunately, all the vaccines developed in the last 20 years have failed to protect Atlantic salmon against P. salmonis [1]. Some intrinsic and extrinsic factors that may explain why commercial vaccines do not provide protection against P. salmonis are: (1) coinfection with sea lice, which can override the protective effects of vaccines [9,10]; (2) host genetic variation, partially protecting some hosts while leaving others unprotected [9,10]; and (3) ineffectiveness in stimulating cellular immunity, which is a key element to protecting against P. salmonis because this bacterium can survive inside the host cells. Likewise, other underlying causes may lead to low vaccine efficacy, such as pathogen genetic variation or a poor match between the vaccine and the circulating strain.
Since outbreaks of Piscirickettsiosis in Chile are mainly caused by two genetic strains of P. salmonis, it has been suggested that this heterogeneity should be considered in vaccine development [11,12]. The reported efficacy of a commercial vaccine would be expected to be low when testing against bacterial strains with low virulence and/or a reduced prevalence in the field [11]. In Chile, two strains—called LF-89 and EM-90—are considered highly and ubiquitously prevalent [13]. These strains show distinct laboratory growth conditions [13] and have major differences in virulence-associated secretion systems and transcriptional profiles [14], resulting in different levels of infectivity [15]. For example, it has been shown that the EM-90-like strain is more aggressive than LF-89, inducing higher cumulative mortalities (EM-90 = 95%; LF-89 = 82%) with a shorter time to death (EM-90 = 42 days; LF-89 = 46 days) in non-vaccinated post-smolts when evaluated by a cohabitation challenge [16,17]. Contrary to the hypothesis of heterogeneity, an experimental vaccine developed from strain EM-90 failed to protect against that same strain [18,19].
In this study, we tested the efficacy of two commercial vaccines against the two most prevalent Chilean genogroups of P. salmonis, LF-89, and EM-90. The first vaccine was a pentavalent bacterin injectable vaccine (AL100005 isolate), and the second was a combination of the pentavalent bacterin vaccine with a live attenuated injectable vaccine (AL 20542 isolate). The cohabitation challenges were carried out with Atlantic salmon (Salmo salar) that were successfully adapted to salt water to best imitate the natural conditions of bacterial infection. In the first trial, LF-89 was evaluated in post-smolt fish given a single infection of P. salmonis, while in the second trial, EM-90-like was evaluated with adult fish in a challenge that included a very low coinfection with sea lice (C. rogercresseyi), to once again better emulate field conditions.
2. Materials and Methods
2.1. Ethics Statement
This work was carried out under the Canadian Council on Animal Care guidance for the care and use of experimental animals. The protocol was approved by the Bioethics Committee of the Pontificia Universidad Católica de Valparaíso and the Comisión Nacional de Investigación Científica y Tecnológica de Chile (FONDECYT No. 1140772). Animals were fed daily ad libitum with a commercial diet. To reduce stress during handling, vaccination was performed on fish sedated with AQUI-S (50% Isoeugenol, 17 mL/100 L water). Fish were euthanized by an overdose of anesthesia (AQUI-S, 50 mL/100 L).
2.2. Commercial Vaccines
The commercial vaccines, hereafter “the vaccine”, used in this study were a pentavalent bacterin vaccine (ALPHA JECT 5-1®; PharmaQ AS, Overhalla, Norway) with antigens against P. salmonis, Vibrio ordalii, Aeromonas salmonicida, IPNV (Infectious Pancreatic Necrosis Virus) and ISAV (Infectious Salmon Anemia Virus) and a monovalent live attenuated vaccine against P. salmonis (ALPHA JECT LiVac® SRS; PharmaQ AS, Overhalla, Norway). This pentavalent vaccine is used by 43% of Chilean farmers (3821 vaccination events over 8884 events in the freshwater phase of production) and thus is the most commonly used vaccine, while the live attenuated vaccine is fifth in terms of usage at 6.8% (608/8884) [8]. The component of these vaccines included for prevention of Piscirickettsiosis is the P. salmonis AL 10005 strain (pentavalent vaccine) and P. salmonis AL 20542 (live attenuated vaccine), respectively. The pentavalent vaccine was used in trial 1, and both vaccines (pentavalent and live attenuated) were given simultaneously in trial 2 as recommended by the manufacturer. No fish was challenged before first completing the time period required for protection indicated by the manufacturer (ALPHA JECT 5-1 = 600 UTA or 46.15 days at 13 °C; ALPHA JECT LiVac = 456 UTA or 35.07 days at 13 °C).
2.3. Challenge with LF-89 Genogroup (Trial 1)
A total of 4987 individually pit-tagged smolt Atlantic salmon were provided in 2017 by Salmones Camanchaca (Puerto Montt, Chile). Fish were transferred to the Neosalmon experimental station (Puerto Montt, Chile) for the cohabitation challenge (Table 1). Smolts were received into a salinity of 6–8 ppt, which was gradually increased over 14 days to 32 ppt. In total, 1002 of the fish previously immunized with the vaccine using the normal production schedule were used as vaccinated fish (342 ± 55 g), while 1062 fish that had been previously injected with Phosphate-Buffered Saline (PBS) were used as unvaccinated fish (314 ± 61 g). The remaining fish were used as Trojan shedders (152 ± 38 g).
Table 1.
Number and proportion of Atlantic salmon used per group and treatment for the first and second trials. In the first trial, post-smolt fish were challenged with the LF-89 genogroup of P. salmonis, while in the second trial, adult fish were challenged with the EM-90 genogroup of P. salmonis and with the sea lice C. rogercresseyi.
| Group | Treatments | First Trial | Second Trial |
|---|---|---|---|
| Cohabitant | Vaccinated (HV) | 496 | 83 |
| Unvaccinated (HUV) | 335 | 96 | |
| Total cohabitant (H) | 831 | 179 | |
| HUV/H | 42% | 53% | |
| Trojan | Total Trojans (T) | 2903 | 183 |
| T/(H + T) | 77% | 41% | |
| Control | Vaccinated (CV) | 506 | 38 |
| Unvaccinated (CUV) | 727 | 42 | |
| Total control (C) | 1233 | 80 | |
| Total fish (H + T + C) | 4987 | 442 |
Vaccinated and unvaccinated fish were distributed into four tanks: two tanks of 15 m3 for the cohabitation challenges and two tanks of 5 m3 for the control without infection. All fish were acclimatized to the experimental conditions (salinity of 32 ppt and a temperature of 15 ± 1 °C) and tanks for at least 15 days prior to the challenge. Further, a health check by RT-PCR was performed by ADL Diagnostic Chile Company (Puerto Montt, Chile) to verify that the fish were free of viral (ISAV and IPNV) and bacterial pathogens (Vibrio ordalii, Flavobacterium psychrophilum, P. salmonis, and Renibacterium salmoninarum). RT-PCR was performed following SERNAPESCA regulations [3]. The cohabitation tanks were challenged by adding Trojan shedders (Table 1, Figure S1A) which had been previously injected with a median lethal dose (LD50 of 1 × 10−2 TCID/mL:TCID: median tissue culture infective dose) of the LF-89 genogroup (isolate PM-38986) provided by ADL Diagnostic Chile (Puerto Montt, Chile). The experiment was conducted for 43 days after the P. salmonis injection of Trojans (Figure S1A). The LD50 used in Trojans was previously determined on 800 fish immunized with the vaccine, which were equally distributed in four treatments and two tanks of 1000 L per treatment. Treatment 1 involved injection with 1 × 10−2 TCID/mL, treatment 2 involved injection with 1 × 10−3 TCID/mL, treatment 3 involved injection with 1 × 10−4 TCID/mL, and treatment 4 involved injection with PBS. Fish were monitored daily for 30 days, and mortalities were recorded.
2.4. Challenge with EM-90-LIKE Genogroup and Coinfection with sea Lice (Trial 2)
A total of 442 individually pit-tagged adult fish were provided in 2019 by Salmones Camanchaca (Puerto Montt, Chile) and transferred to the Aquadvice experimental station (Puerto Montt, Chile) for the cohabitation challenge (Table 1). In total, 121 of the fish, which had been previously immunized with the vaccine using the normal production schedule, were used as vaccinated fish (1294 ± 326 g), while 138 that had been previously injected with PBS were used as unvaccinated fish (1228 ± 345 g). The remaining fish were used as Trojan shedders (1308 ± 337 g).
Vaccinated and unvaccinated fish were distributed into three tanks of 11 m3: two tanks for the cohabitation challenges and one tank for the control without infection. All fish were acclimatized to the experimental conditions (salinity of 32 ppt and a temperature of 15 ± 1 °C) for at least 15 days prior to the challenge. Further, a health check by RT-PCR was performed by ADL Diagnostic Chile Company (Puerto Montt, Chile) to verify that the fish were free of viral (ISAV and IPNV) and bacterial pathogens (Vibrio ordalii, Flavobacterium psychrophilum, P. salmonis, and Renibacterium salmoninarum). The cohabitation tanks were challenged by adding Trojan shedders (Table 1, Figure S1B) which had been previously injected with a median lethal dose (LD50) of 1 × 10−3.5 TCID/mL of the EM-90 genogroup (isolate PS03-04) provided by Fraunhofer Chile (Santiago, Chile). Seven days after the Trojan fish were challenged with P. salmonis, all fish (cohabitant, Trojan and control) were infested with C. rogercresseyi copepodids. The coinfection procedure was established based on our previous studies [20], but in this case a low infection rate was applied to mimic the natural infection rates normally seen in field conditions [21]. Infections with sea lice were performed by adding 20 copepodites per fish to each control and coinfection tank. Copepodites were collected from egg-bearing females reared in the laboratory and confirmed to be pathogen-free (P. salmonis, R. salmoninarum, IPNV, and ISAV) by RT-PCR. After the addition of parasites, water flow was stopped for a period of 8 h, and tanks were covered to decrease light intensity, which favors the successful settlement of sea lice on fish [20]. Parasite counts were performed a week after the infestation for nine fish per tank. The challenge lasted 60 days after the Trojans’ infection with P. salmonis.
The LD50 used in Trojans was previously determined on 330 immunized fish, which were equally distributed in five treatments and two tanks of 720 L per treatment. Treatment 1 involved injection with 1 × 10−1.5 TCID/mL, treatment 2 involved injection with 1 × 10−2.5 TCID/mL, treatment 3 involved injection with 1 × 10−3.5 TCID/mL, treatment 4 involved injection with 1 × 10−4.5 TCID/mL, and treatment 5 involved injection with PBS. Fish were monitored daily for 30 days, and mortalities were recorded (Figure S1B).
2.5. Necropsy Analysis
Macroscopic lesions from 10 controls and 10 cohabitant fish in each trial were analyzed. Two different veterinarians who were blinded to the treatments studied fresh samples from trials 1 or 2. In the challenge with LF-89 genogroup, macroscopic lesions in the liver were evaluated at 21 days post-infection, where vacuolar degeneration, hepatitis, and hepatocyte atrophy were described according to their presence or absence. Additionally, 47 vaccinated and unvaccinated fish from cohabitation and control tanks were analyzed by immunohistochemistry to detect the presence or absence of P. salmonis in the liver both 21 days after the challenge and at the end of the experiment. For the EM-90 genogroup experiment, pathological signs were evaluated only at the end of the challenge; this analysis included the presence or absence of nodules in the liver, congestive liver, and hepatomegaly.
2.6. ELISA
An indirect Enzyme-Linked Immunosorbent Assay (ELISA) was performed in serum samples from the first trial only—the fish challenged with the LF-89 genogroup. Secretion levels of total immunoglobulin (Igs), antigen-specific immunoglobulins against P. salmonis (spIgs), tumor necrosis factor-alpha (Tnfα) and interferon-gamma (Ifnγ) were measured following the protocol of Morales-Lange et al. [22]. Briefly, the total protein concentration of each sample was determined by the BCA (Bicinchoninic acid) method (Pierce, Thermo Fisher, Waltham, MA, USA) according to the supplier’s instructions. Then, each sample was diluted in carbonate buffer (60 mM NaHCO3, pH 9.6), seeded in duplicate at 50 ng µL−1 (100 µL) in a Maxisorp plate (Nunc, Thermo Fisher Scientific, Waltham, USA) and incubated overnight at 4 °C. After that, the plates were blocked with 200 µL per well of 1% Bovine Serum Albumin (BSA) for 2 h at 37 °C, and later the primary antibodies (Table S1 and Figure S2) [23] were added for 90 min at 37 °C. Next, a secondary antibody—HRP (Thermo Fisher)—was added for a 60 min incubation at 37 °C at a 1:7000 dilution. Finally, 100 µL per well of chromogen substrate 3,30,5,50-tetramethylbenzidine (TMB) single solution (Invitrogen, Carlsbad, CA, USA) was added and the plates were incubated for 30 min at room temperature. The reaction was stopped with 50 µL of 1 N sulfuric acid and read at 450 nm on a VERSAmax microplate reader (Molecular Devices, San Jose, CA, USA). For the detection of spIg, 50 ng µL−1 of total protein extract from P. salmonis [24] were seeded per well in a Maxisorp plate (diluted in 100 µL of carbonate buffer) and incubated overnight at 4 °C. After blocking with 1% BSA (200 µL per well), each fish serum sample was incubated in duplicate at a total Igs concentration of 50 ng µL−1 for 90 min at 37 °C. After that, the ELISA protocol described above was followed.
2.7. Statistical Analysis
The mortality was recorded, and data were represented using Kaplan–Meier survival curves [25]. The protection elicited by vaccines was determined by comparing the survival percentage of vaccinated and unvaccinated groups using a Log-rank test. Further, the Relative Proportion Survival (RPS) was calculated as
| RPS (%) = (1−A/B) ∗ 100 |
where A and B are the mortalities at the end of challenges in vaccinated and unvaccinated fish, respectively.
Additionally, differences in the pathological symptoms of P. salmonis infection between different treatments were analyzed using a non-parametric Chi-square test. Finally, significant differences in ELISA tests were compared using the Student’s two-tailed t-test, p < 0.05. All statistical analyses were performed using R Core Team (RStudio, Vienna, Austria). Graphs were designed with GraphPad Prism 8.0 software (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Vaccine Efficacy against the LF-89 Genogroup
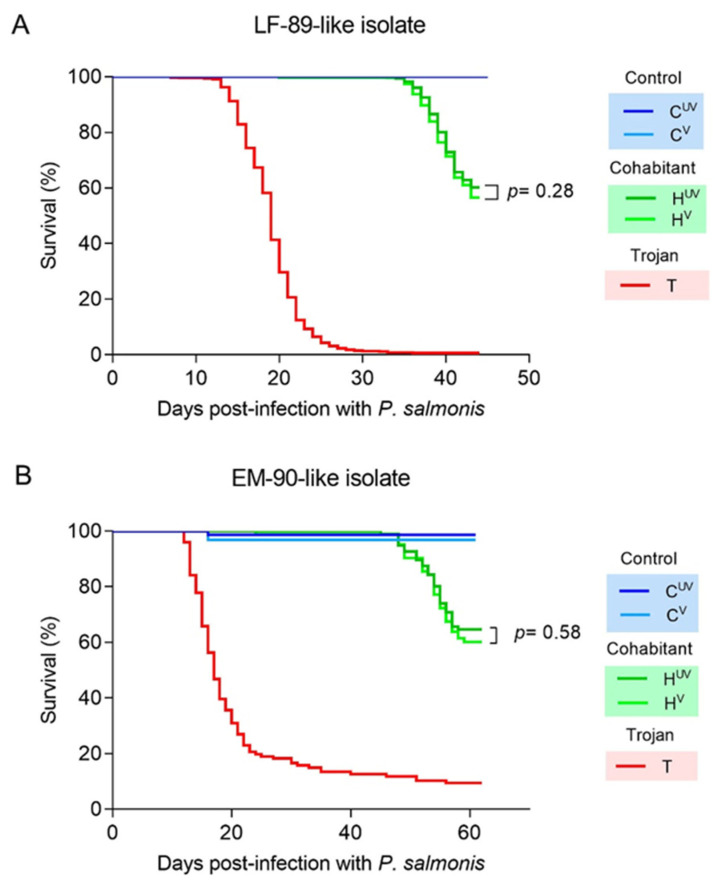
No mortality was recorded in the non-infected control fish. However, the cohabitation challenge with the LF-89 strain resulted in high mortality in the experimental fish. There was no evidence that the pentavalent vaccine generated effective protection against the LF-89 genogroup. The vaccine delayed mortalities by two days (HUV: 34 dpi and HV: 36 dpi), but both unvaccinated and vaccinated fish showed similar survival during and at the end of the challenges (HUV: 60.3% and HV: 56.7%, Figure 1A). Therefore, the survival test did not reveal significant differences between vaccinated and unvaccinated treatments (p = 0.28).
Figure 1.
Survival curves: (A) Single infection of Atlantic salmon post-smolt with the P. salmonis LF-89 genogroup. (B) Coinfection of Atlantic salmon adults with the P. salmonis EM-90 genogroup and the sea louse C. rogercresseyi. Fish from the first trial were immunized with pentavalent injectable vaccine, and fish from the second trial with pentavalent injectable plus monovalent live attenuated injectable. Abbreviations: CUV: control unvaccinated; CV: control vaccinated; HUV: cohabitant unvaccinated; HV: cohabitant vaccinated; T: Trojan.
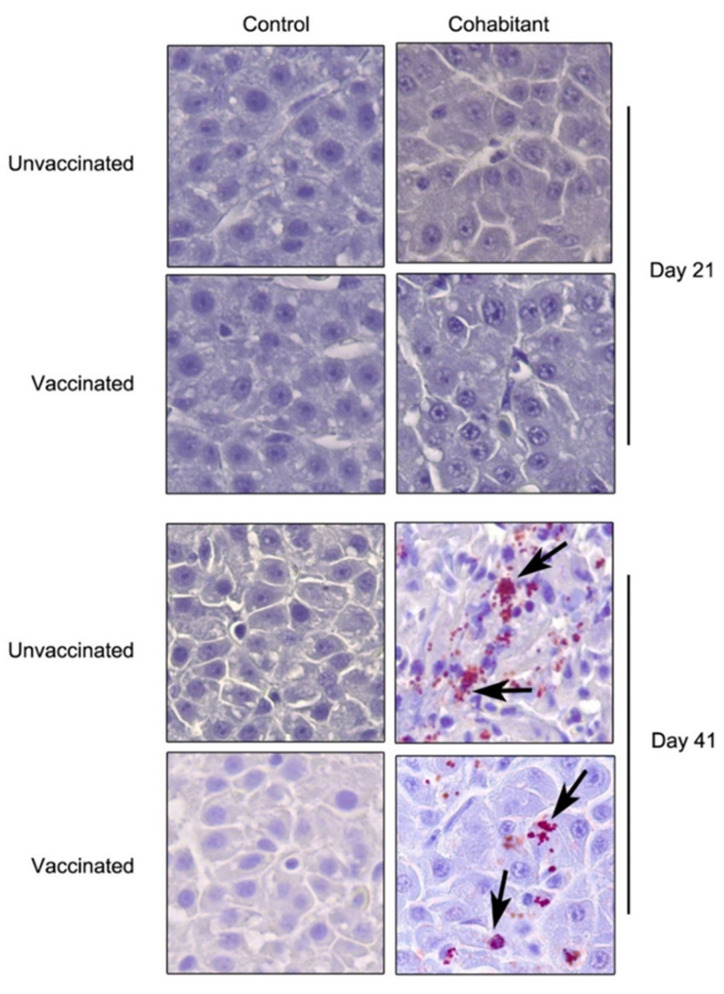
Dead fish and large numbers of vaccinated and unvaccinated live fish at the end of the challenge showed multiple hemorrhagic ulcers on the skin typical of a severe P. salmonis infection. P. salmonis infection was also evident in the liver of both vaccinated and unvaccinated fish at the end of the challenge, but not at 21 days after infection (Figure 2). On the other hand, vaccination increased the presence of hepatocyte atrophy in comparison with unvaccinated fish in the control treatment at 21 days post-infection (Table 2). A similar trend was observed in the cohabitant fish, but without significant differences (Table 2). Once the challenge was over, the fish were evaluated for most common salmon diseases, revealing the appearance of secondary infections of Piscine orthoreovirus (Figure S3) and Tenacibaculum dicentrarchi in some animals.
Figure 2.
Presence of LF-89 genogroup of P. salmonis (black arrows) in liver samples of Atlantic salmon. Piscirickettsiosis was detected in 11 out of 47 fish analyzed by immunohistochemistry—magnification 63X.
Table 2.
Pathological signs in Atlantic salmon challenged with the LF-89 genogroup of P. salmonis at day 21 post-infection in cohabitant and control groups. Differences between vaccinated and unvaccinated fish were evaluated with a Chi-squared statistical test (* = p < 0.05). Abbreviations: UV: unvaccinated fish and V: vaccinated fish.
| Group | Pathological Signs | Presence of Pathological Signs | Treatment | Proportion | Chi-Square Test | |||
|---|---|---|---|---|---|---|---|---|
| UV | V | UV | V | X 2 | p-Value | |||
| Cohabitant | Vacuolar | No | 2 | 4 | 0.2 | 0.4 | 0.24 | 0.63 |
| degeneration | Yes | 8 | 6 | 0.8 | 0.6 | |||
| Total | 10 | 10 | ||||||
| Hepatitis | No | 9 | 9 | 0.9 | 0.9 | 0 | 1 | |
| Yes | 1 | 1 | 0.1 | 0.1 | ||||
| Total | 10 | 10 | ||||||
| Hepatocyte | No | 8 | 4 | 0.8 | 0.4 | 1.88 | 0.17 | |
| atrophy | Yes | 2 | 6 | 0.2 | 0.6 | |||
| Total | 10 | 10 | ||||||
| Control | Vacuolar | No | 1 | 3 | 0.1 | 0.3 | 0.31 | 0.58 |
| degeneration | Yes | 9 | 7 | 0.9 | 0.7 | |||
| Total | 10 | 10 | ||||||
| Hepatitis | No | 8 | 7 | 0.8 | 0.7 | 0 | 1 | |
| Yes | 2 | 3 | 0.2 | 0.3 | ||||
| Total | 10 | 10 | ||||||
| Hepatocyte | No | 10 | 5 | 1 | 0.5 | 4.27 | <0.05 * | |
| atrophy | Yes | 0 | 5 | 0 | 0.5 | |||
| Total | 10 | 10 | ||||||
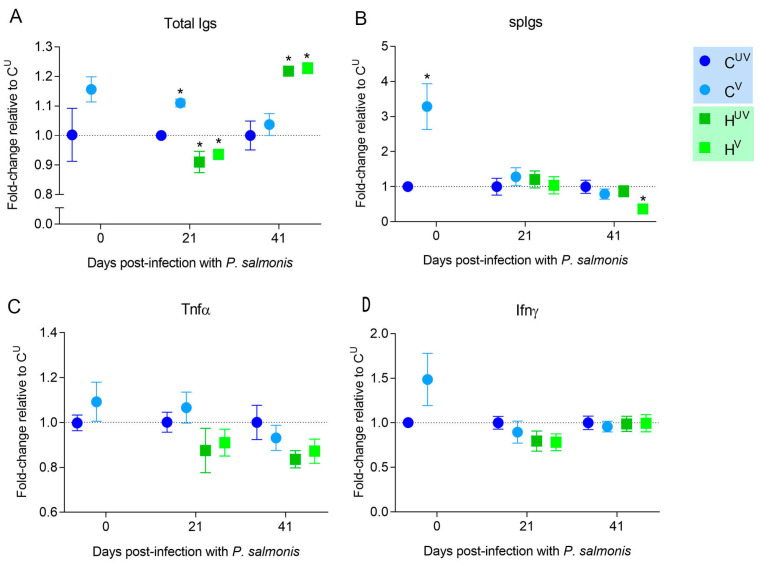
Serum samples showed a significant increase of total Igs at 21 days post-infection (Figure 3A) in the control group of vaccinated fish (CV). However, at the same sampling time, both unvaccinated and vaccinated experimental fish showed a decrease in total Igs levels. This trend was reversed at 41 dpi, since both groups (HUV and HV) significantly increased their levels of total Igs. On the other hand, when specific immunoglobulins against P. salmonis were measured (Figure 3B), an increase was detected in CV even before the challenge with P. salmonis. Nevertheless, after 41 days post-infection, HV group showed lower titers of P. salmonis spIgs than the other groups. Finally, the evaluation of Tnfα and Ifnγ secretion did not show significant changes between treatments (Figure 3C,D).
Figure 3.
Protein levels by ELISA: Secretion of total Igs (A), antigen specific Igs (B), tumor necrosis factor alpha (Tnfα) (C), and interferon gamma (Ifnγ) (D) in serum samples from Atlantic salmon measured by ELISA after a challenge with P. salmonis in the first trial (single infection of the LF-89 genogroup). Fish immunized with pentavalent injectable vaccine. Data represent the mean ± SEM (n = 10). Significant differences compared to CUV by Student t-test two-tailed (* = p < 0.05). Abbreviations: CUV: control unvaccinated; CV: control vaccinated; HUV: cohabitant unvaccinated; HV: cohabitant vaccinated.
3.2. Vaccine Efficacy against the EM-90 Genogroup with Low Sea Lice Coinfection
In the second trial, adult fish were coinfected with sea lice to mimic natural conditions in the field. Seven days after sea lice infestation, the prevalence of sea lice was 100% in treatment and control tanks, with no significant differences in the abundance of the parasites between tanks (Tank 1 = 10.4 ± 4.0; Tank 2 = 11.7 ± 3.0; Control tank = 9.7 ± 6.6). The vaccine (pentavalent injectable + monovalent live attenuated injectable) was not able to protect against the EM-90 genogroup (Survival percent: HV: 60.2% and HUV: 64.6%; Figure 1B; p = 0.58) in cohabitant fish with low-level sea lice infection. However, a small effect of delayed mortalities was observed; for example, steady mortality started three days later for vaccinated fish compared with unvaccinated fish (HV: 48 dpi and HUV: 45 dpi). The control tank infected only with sea lice experienced very low mortality, with one in the unvaccinated fish (CUV) and two in the vaccinated fish (CV).
Vaccinated and unvaccinated mortalities showed hemorrhagic ulcers on the skin typical of a severe P. salmonis infection. Further, when we compared cohabitant and control fish at the end of the challenges, infection with P. salmonis was evident in the cohabitant fish through the three evaluated pathological signs: nodules in liver, congestive liver, and hepatomegaly (Table 3). However, we did not find differences between vaccinated and unvaccinated fish in cohabitant fish (Table 3). For instance, in the cohabitant treatment, nine unvaccinated fish presented a congestive liver, compared to 10 vaccinated fish showing that symptom. Similar patterns were found for nodules in the liver and hepatomegaly.
Table 3.
Pathological signs in Atlantic salmon challenged with the EM-90 genogroup of P. salmonis and infestation with C. rogercresseyi at day 47–51 post-infection in cohabitant and control groups. Differences between vaccinated and unvaccinated fish were evaluated with a Chi-squared statistical test. Abbreviations: UV: unvaccinated fish and V: vaccinated fish.
| Group | Pathological Signs | Presence of Pathological Signs | Treatment | Proportion | Chi-Square Test | |||
|---|---|---|---|---|---|---|---|---|
| UV | V | UV | V | X 2 | p-Value | |||
| Cohabitant | Nodules in | No | 0 | 0 | 0 | 0 | 0 | 1 |
| liver | Yes | 10 | 10 | 1 | 1 | |||
| Total | 10 | 10 | ||||||
| Congestive | No | 1 | 0 | 0.1 | 0 | 0.02 | 0.96 | |
| liver | Yes | 9 | 10 | 0.9 | 1 | |||
| Total | 10 | 10 | ||||||
| Hepatomegaly | No | 0 | 0 | 0 | 0 | 0 | 1 | |
| Yes | 10 | 10 | 1 | 1 | ||||
| Total | 10 | 10 | ||||||
| Control | Nodules in | No | 10 | 10 | 1 | 1 | 0 | 1 |
| liver | Yes | 0 | 0 | 0 | 0 | |||
| Total | 10 | 10 | ||||||
| Congestive | No | 10 | 9 | 1 | 0.9 | 0 | 1 | |
| liver | Yes | 0 | 1 | 0 | 0.1 | |||
| Total | 10 | 10 | ||||||
| Hepatomegaly | No | 10 | 9 | 1 | 0.9 | 0 | 1 | |
| Yes | 0 | 1 | 0 | 0.1 | ||||
| Total | 10 | 10 | ||||||
4. Discussion
Vaccination is one of the most used strategies to prevent and control diseases in aquaculture [26,27]. However, vaccines have failed to control and prevent Piscirickettsiosis, for reasons that remain elusive [1,27,28,29]. This manuscript evaluated whether the heterogeneity of P. salmonis could explain the low vaccine efficacy of a commercial vaccine whose active principle is a bacterin developed using the P. salmonis AL 10005 strain and a live attenuated vaccine developed with AL 20542 isolates. To achieve this, we evaluated the vaccine efficacy using the two most prevalent and ubiquitous genetic variants of P. salmonis in Chile. Challenges were designed to mimic the natural conditions of infection; thus, LF-89 was evaluated with post-smolt fish in a single infection of P. salmonis, and EM-90 was evaluated with adult fish in a challenge that included a very low coinfection with the sea louse C. rogercresseyi. In this study, we found no evidence that vaccines developed with the P. salmonis AL 10005 or AL 20542 isolates confer protection against infection caused by the LF-89 or EM-90 genogroups in Atlantic salmon.
The absent or low level of protection provided by the commercial vaccines against infection caused by P. salmonis in the field could be related to the use of a model for the evaluation of protection in the product development vaccination trials that is not reproducing real field conditions. For example, the route of infection has been proposed as a relevant factor in the performance of a vaccine. Here, we selected a cohabitation model of challenges, because this best mimics the natural infection route [30]. On the other hand, several studies evaluating vaccine efficacy against P. salmonis have been performed by intraperitoneal injection [31,32,33,34]. Intraperitoneal injection is preferred because it is a synchronized and effective infection route that shortens the time to produce disease symptoms, decreasing the cost of trials [18,19]. Vaccine efficacy has previously been found to be affected by the route of infection for furunculosis [35] but not for Piscirickettsiosis in Atlantic salmon [19].
Moreover, coinfection with other pathogens such as sea lice is usually not considered in the evaluation of P. salmonis vaccine efficacy in laboratory-controlled conditions. We consider that this overestimates the true ability of vaccines to control Piscirickettsiosis for three reasons: first, sea lice are highly prevalent in the ocean; second, the long culture times in the sea ensure that fish will be infected not once but several times by this parasite; third, it has been shown that sea lice infection can override the protective effects of vaccination [9]. We observed no pathological signs associated with P. salmonis in the control tank, and mortality was significantly lower in the control tanks (less than 2%; 3 of 137 fish) than in the coinfection treatment animals (36–40%). Because we did not observe differences in mortality or pathological signs between vaccinated and unvaccinated adult fish in the cohabitation experiment we predict that the evaluated vaccine will not protect fish in the field.
The immune mechanisms involved in vaccine protection against P. salmonis are poorly understood. In this research, the vaccine was able to induce an increase of spIgs in vaccinated fish. However, this occurred before the challenge with P. salmonis. After the challenge, cohabiting fish showed increases only in total Igs (41 dpi) and even a decrease of spIgs against P. salmonis by 41 dpi, perhaps due to B cell depletion. Apparently, the vaccine is not able to activate components of acquired immunity such as specific antibodies or cytokines associated with TH1 profiles (Tnfα and Ifnγ) once fish face P. salmonis infection, perhaps because P. salmonis is an intracellular parasite that requires a TH2 response that inhibits TH1 responses. This suggests that the vaccine could act as an immunostimulant for the adaptive response at early time points, but not as a vaccine that induces future specific secondary responses. It has already been reported that vaccines may induce weaker or shorter-lived immunity in fish, mainly due to the low immunogenicity of the antigens used or because they cannot modulate the antigen presentation processes effectively during the different stages of immunity [36]. Therefore, the protective mechanism that P. salmonis vaccines might have in the field [8] needs to be clarified.
In Chile, the Agricultural and Livestock Service of Chile (SAG) authorized P. salmonis vaccines that meet a minimum protection of ≥70% RPS in experimental trials to be marketed. However, there is little evidence of their effectiveness under field conditions [8]. In this study, the minimum protection of ≥70% RPS was not reproduced either against P. salmonis LF-89 genogroup or in the EM-90 genogroup. Unfortunately, neither the pharmaceutical companies nor the SAG (Agricultural and Livestock Service) publicly release the results of efficacy studies that authorize the marketing of vaccines in Chile. This prevented us from comparing our results with the efficacy studies carried out by pharmaceutical companies. Vaccine efficacy studies must be public and must consider both the genetic heterogeneity of the host and the pathogen’s heterogeneity. In fact, we do not know whether pathogen heterogeneity was considered or if the most vulnerable populations of fish were included when the efficacy of the Piscirickettsiosis vaccine was evaluated by the SAG, as is recommended by the World Organization for Animal Health (OIE).
5. Conclusions
Commercial vaccines against P. salmonis have failed to reduce mortality or prevent outbreaks in field conditions. In this study, we found no evidence that commercial vaccines confer protection in Atlantic salmon against the LF-89 or EM-90 genogroups of P. salmonis. We have provided insights into the heterogeneity of P. salmonis that could explain the low efficacy of commercial vaccines whose active agent is different to the two most prevalent and ubiquitous naturally occurring genetic variants of P. salmonis in Chile.
Acknowledgments
We wish to thank all of the staff from the salmon selective breeding program at Salmones Camanchaca, with special thanks to Darwin Muñoz, Sonia Velazquez and Sergio Navarro for their professional support and collaboration in the experimental work. We would like to thank Salmones Camanchaca for providing fish, materials and logistics to perform this study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology11070993/s1, Figure S1: Experimental pipeline (A) Single infection of Atlantic salmon post-smolt with the P. salmonis LF 89-like genogroup. (B) Coinfection of Atlantic salmon adults with the P. salmonis EM-90-like genogroup and the sea louse C. rogercresseyi. Table S1: Primary polyclonal antibodies used in ELISA analysis; Figure S2: Validation of antibodies against total serum immunoglobulins (Igs) of Salmo salar: (A) Indirect ELISA calibration curve between total serum Igs concentration of S. salar (ng µL−1) and optical density at 450 nm; (B) Western blot. Antibodies were produced in mice using total serum Igs from Atlantic salmon as antigen. The antigen was obtained by the caprylic acid technique for immunoglobulin purification [23]; Figure S3: Presence of Piscine orthoreovirus (black arrows) in heart samples of Atlantic salmon from the first trial at day 41 post infection with LF-89 genogroup of P. salmonis. The virus was detected in 7 out of 17 fish analyzed by immunohistochemistry—magnification 63X.
Author Contributions
Conceptualization, C.F., B.D., P.C., G.S., C.S. and J.A.G.; Data curation, D.T.; Formal analysis, C.F., D.T. and J.A.G.; Funding acquisition, P.C. and J.A.G.; Investigation, C.F., B.M.-L., L.M., G.S., C.S. and J.A.G.; Supervision, J.A.G.; Writing—original draft, D.T. and J.A.G.; Writing—review and editing, C.F., B.M.-L., L.M., B.D. and P.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The animal study protocol was approved by the Bioethics Committee of the Pontificia Universidad Católica de Valparaíso and the Comisión Nacional de Investigación Científica y Tecnológica de Chile (FONDECYT No. 1140772).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the reported results can be found in this GitHub repository: https://github.com/GenomicsLaboratory/ReproducibleResearch (accessed on 12 May 2022).
Conflicts of Interest
We declare that J.A.G., L.M. and P.C. provided genetic and immunological services to different salmon companies in Chile during the execution of this experiment. G.S. and C.S. were employed in Salmones Camanchaca (Puerto Montt, Chile) during the execution of this research. C.F., D.T., B.M.-L. and B.D. declare no competing financial interest.
Funding Statement
This research study was funded by CONICYT-Chile through the project FONDECYT No. 1140772 awarded to J.A.G. and P.C. Furthermore, J.A.G. was supported by the Cooperative Research Program Fellowships of OECD—PCI 2015-CONICYT. C.F. was supported by PUCV and CONICYT-Chile through a Postdoctoral fellowship (Proyecto VRIEA-PUCV Postdoctorado and FONDECYT No. 3170744). D.T. was supported by ANID-Chile through a Postdoctoral fellowship (Fondecyt No. 3210502). B.M.-L. was supported by the Postdoctoral program from the National Research and Development Agency of Chile (ANID-Chile No. 74200139).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maisey K., Montero R., Christodoulides M. Vaccines for piscirickettsiosis (salmonid rickettsial septicaemia, SRS): The Chile perspective. Expert Rev. Vaccines. 2017;16:215–228. doi: 10.1080/14760584.2017.1244483. [DOI] [PubMed] [Google Scholar]
- 2.Rozas M., Enriquez R. Piscirickettsiosis and Piscirickettsia salmonis in fish: A review. J. Fish Dis. 2014;37:163–188. doi: 10.1111/jfd.12211. [DOI] [PubMed] [Google Scholar]
- 3.SERNAPESCA . Informe Sanitario Salmonicultura en Centros Marinos 2019. Servicio Nacional de Pesca y Acuicultura; Santiago, Chile: 2020. pp. 1–38. [Google Scholar]
- 4.Brocklebank J., Ebelyn T., Speare D.J., Armstrong R. Rickettsial septicemia in farmed Atlantic and chinook salmon in British Columbia: Clinical presentation and experimental transmission. Can. Vet. J. 1993;34:745–748. [PMC free article] [PubMed] [Google Scholar]
- 5.Grant A.N., Brown A.G., Cox D.I., Birkbeck T.H., Griffen A.A. Rickettsia-like organism in farmed salmon. Vet. Rec. 1996;138:423. [PubMed] [Google Scholar]
- 6.Olsen A.J., Melby H., Speilberg L., Evensen O., Hastein T. Piscirickettsia salmonis infection in Atlantic salmon Salmo salar in Norway-epidemiological, pathological and microbiological findings. Dis. Aquat. Org. 1997;31:35–48. doi: 10.3354/dao031035. [DOI] [Google Scholar]
- 7.SERNAPESCA . Informe Sanitario de Salmonicultura en Centros Marinos 2016. Servicio Nacional de Pesca y Acuicultura; Santiago, Chile: 2017. pp. 1–37. [Google Scholar]
- 8.Happold J., Sadler R., Meyer A., Hillman A., Cowled B., Mackenzie C., Gallardo Lagno A.L., Cameron A. Effectiveness of vaccination for the control of salmonid rickettsial septicaemia in commercial salmon and trout farms in Chile. Aquaculture. 2020;520:734968. doi: 10.1016/j.aquaculture.2020.734968. [DOI] [Google Scholar]
- 9.Figueroa C., Bustos P., Torrealba D., Dixon B., Soto C., Conejeros P., Gallardo J.A. Coinfection takes its toll: Sea lice override the protective effects of vaccination against a bacterial pathogen in Atlantic salmon. Sci. Rep. 2017;7:17817. doi: 10.1038/s41598-017-18180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figueroa C., Veloso P., Espin L., Dixon B., Torrealba D., Elalfy I.S., Afonso J.M., Soto C., Conejeros P., Gallardo J.A. Host genetic variation explains reduced protection of commercial vaccines against Piscirickettsia salmonis in Atlantic salmon. Sc. Rep. 2020;10:18252. doi: 10.1038/s41598-020-70847-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nourdin-Galindo G., Sanchez P., Molina C.F., Espinoza-Rojas D.A., Oliver C., Ruiz P., Vargas-Chacoff L., Carcamo J.G., Figueroa J.E., Mancilla M., et al. Comparative Pan-Genome Analysis of Piscirickettsia salmonis Reveals Genomic Divergences within Genogroups. Front. Cell. Infect. Microbiol. 2017;7:459. doi: 10.3389/fcimb.2017.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otterlei A., Brevik O.J., Jensen D., Duesund H., Sommerset I., Frost P., Mendoza J., McKenzie P., Nylund A., Apablaza P. Phenotypic and genetic characterization of Piscirickettsia salmonis from Chilean and Canadian salmonids. BMC Vet. Res. 2016;12:55. doi: 10.1186/s12917-016-0681-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saavedra J., Hernandez N., Osses A., Castillo A., Cancino A., Grothusen H., Navas E., Henriquez P., Bohle H., Bustamante F., et al. Prevalence, geographic distribution and phenotypic differences of Piscirickettsia salmonis EM-90-like isolates. J. Fish Dis. 2017;40:1055–1063. doi: 10.1111/jfd.12581. [DOI] [PubMed] [Google Scholar]
- 14.Millar A., Tapia P., Gomez F., Marshall S., Fuentes D., BValdes J. Draft genomes and reference transcriptomes extend the coding potential of the fish pathogen Piscirickettsia salmonis. Electron. J. Biotechnol. 2018;33:36–38. doi: 10.1016/j.ejbt.2018.04.002. [DOI] [Google Scholar]
- 15.Bohle H., Hemríquez P., Grothusen H., Navas E., Sandoval A., Bustamante F., Bstos P., Mancilla M. Comparative Genome Analysis of Two Isolates of the Fish Pathogen Piscirickettsia salmonis from Different Hosts Reveals Major Differences in Virulence-Associated Secretion Systems. Genomea. 2014;2:e01219-14. doi: 10.1128/genomeA.01219-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rozas-Serri M., Ildefonso R., Pena A., Enriquez R., Barrientos S., Maldonado L. Comparative pathogenesis of piscirickettsiosis in Atlantic salmon (Salmo salar L.) post-smolt experimentally challenged with LF-89-like and EM-90-like Piscirickettsia salmonis isolates. J. Fish Dis. 2017;40:1451–1472. doi: 10.1111/jfd.12671. [DOI] [PubMed] [Google Scholar]
- 17.Rozas-Serri M., Pena A., Arriagada G., Enriquez R., Maldonado L. Comparison of gene expression in post-smolt Atlantic salmon challenged by LF-89-like and EM-90-like Piscirickettsia salmonis isolates reveals differences in the immune response associated with pathogenicity. J. Fish Dis. 2018;41:539–552. doi: 10.1111/jfd.12756. [DOI] [PubMed] [Google Scholar]
- 18.Cardella M.A., Eimers M.E. Safety and Potency Testing of Federally Licensed Fish Bacterins. J. Aquat. Anim. Health. 1990;2:49–55. doi: 10.1577/1548-8667(1990)002<0049:SAPTOF>2.3.CO;2. [DOI] [Google Scholar]
- 19.Meza K., Inami M., Dalum A.S., Lund H., Bjelland A.M., Sorum H., Lovoll M. Comparative evaluation of experimental challenge by intraperitoneal injection and cohabitation of Atlantic salmon (Salmo salar L) after vaccination against Piscirickettsia salmonis (EM90-like) J. Fish Dis. 2019;42:1713–1730. doi: 10.1111/jfd.13091. [DOI] [PubMed] [Google Scholar]
- 20.Araya A., Mancilla M., Lhorente J.P., Neira R., Gallardo J.A. Experimental challenges of Atlantic salmon Salmo salar with incremental levels of copepodids of sea louse Caligus rogercresseyi: Effects on infestation and early development. Aquacult. Res. 2012;43:1904–1908. doi: 10.1111/j.1365-2109.2011.02991.x. [DOI] [Google Scholar]
- 21.Bravo S., Treasurer J., Sepulveda M., Lagos C. Effectiveness of hydrogen peroxide in the control of Caligus rogercresseyi in Chile and implications for sea louse management. Aquaculture. 2010;303:22–27. doi: 10.1016/j.aquaculture.2010.03.007. [DOI] [Google Scholar]
- 22.Morales-Lange B., González-Aravena M., Font A., Guzmán F., Mercado L. Detection of peroxiredoxin-like protein in Antarctic sea urchin (Sterechinus neumayeri) under heat stress and induced with pathogen-associated molecular pattern from Vibrio anguillarum. Polar Biol. 2018;41:2065–2073. doi: 10.1007/s00300-018-2346-x. [DOI] [Google Scholar]
- 23.Fishman J.B., Berg E.A. Preparation of Antibody Using Caprylic Acid. Cold Spring Harb. Protoc. 2018;2018:475–476. doi: 10.1101/pdb.prot099127. [DOI] [PubMed] [Google Scholar]
- 24.Carril G., Gómez F., Marshall S. Expression of flagellin and key regulatory flagellar genes in the non-motile bacterium Piscirickettsia salmonis. Dis. Aquat. Org. 2017;123:29–43. doi: 10.3354/dao03079. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan E.L., Meier P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958;53:457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 26.Assefa A., Abunna F. Maintenance of Fish Health in Aquaculture: Review of Epidemiological Approaches for Prevention and Control of Infectious Disease of Fish. Veter. Med. Int. 2018;2018:5432497. doi: 10.1155/2018/5432497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams A. Progress, challenges and opportunities in fish vaccine development. Fish Shellfish Immunol. 2019;90:210–214. doi: 10.1016/j.fsi.2019.04.066. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez C.A., Gomez F.A., Mercado L., Ramirez R., Marshall S.H. Piscirickettsia salmonis Imbalances the Innate Immune Response to Succeed in a Productive Infection in a Salmonid Cell Line Model. PloS OnE. 2016;11:e0163943. doi: 10.1371/journal.pone.0163943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cabello F.C., Godfrey H.P. Salmon aquaculture, Piscirickettsia salmonis virulence, and One Health: Dealing with harmful synergies between heavy antimicrobial use and piscine and human health. Aquaculture. 2019;507:451–456. doi: 10.1016/j.aquaculture.2019.04.048. [DOI] [Google Scholar]
- 30.Nordmo R. Strengths and weaknesses of different challenge methods. Dev. Biol. Standard. 1997;90:303–309. [PubMed] [Google Scholar]
- 31.Kuzyk M., Burian J., Machander D., Dolhaine D., Cameron S., Thornton J., Kay W. An efficacious recombinant subunit vaccine against the salmonid rickettsial pathogen Piscirickettsia salmonis. Vaccine. 2001;19:2337–2344. doi: 10.1016/S0264-410X(00)00524-7. [DOI] [PubMed] [Google Scholar]
- 32.Salonius K., Siderakis C., Mackinnon A., Griffiths S. Use of Arthrobacter davidanieli as a live vaccine against Renibacterium salmoninarum and Piscireckettsia salmonis in salmonis. Prog. Fish Vaccinol. 2005;121:189–197. [PubMed] [Google Scholar]
- 33.Tobar J.A., Jerez S., Caruffo M., Bravo C., Contreras F., Bucarey S.A., Harel M. Oral vaccination of Atlantic salmon (Salmo salar) against salmonid rickettsial septicaemia. Vaccine. 2011;29:2336–2340. doi: 10.1016/j.vaccine.2010.12.107. [DOI] [PubMed] [Google Scholar]
- 34.Wilhelm V., Miquel A., Burzio L.O., Rosemblatt M., Engel E., Valenzuela S., Parada G., Valenzuela P.D. A vaccine against the salmonid pathogen Piscirickettsia salmonis based on recombinant proteins. Vaccine. 2006;24:5083–5091. doi: 10.1016/j.vaccine.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 35.Midtlyng P. Critical assessment of regulatory standards and tests for fi sh vaccines. Dev. Biol. 2005;121:219–226. [PubMed] [Google Scholar]
- 36.Rozas-Serri M., Pena A., Maldonado L. Gene expression associated with immune response in Atlantic salmon head-kidney vaccinated with inactivated whole-cell bacterin of Piscirickettsia salmonis and pathogenic isolates. Fish Shellfish Immunol. 2019;93:789–795. doi: 10.1016/j.fsi.2019.08.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the reported results can be found in this GitHub repository: https://github.com/GenomicsLaboratory/ReproducibleResearch (accessed on 12 May 2022).