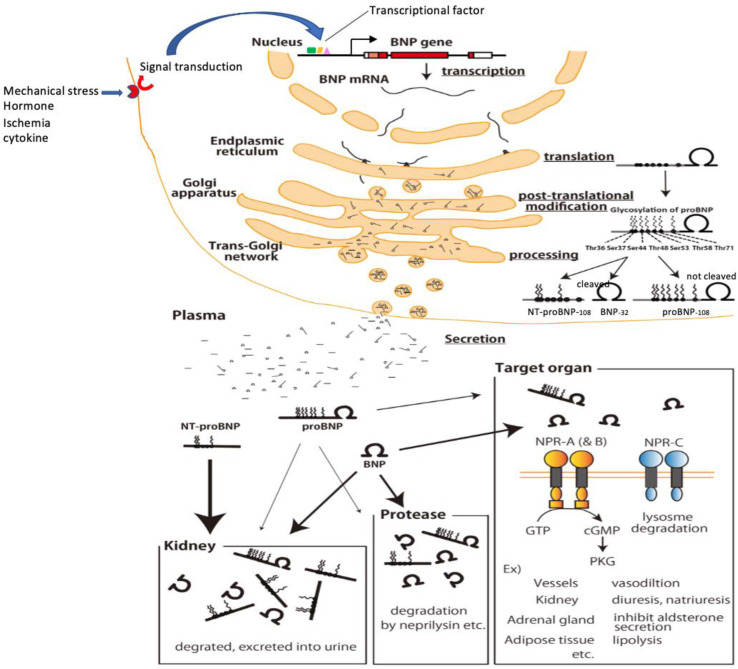
Figure 4.
Production, processing, secretion and metabolism of BNP in cardiomyocytes. Mechanical stress, ischemia, hormones, cytokines, etc. stimulate the receptors and enhance BNP gene expression via signal transduction/transcription factors. The enhanced BNP mRNA, which undergoes splicing, crosses the nuclear membrane and is translated in the endoplasmic reticulum to produce preproBNP. The signal peptide is then removed by signalpeptidase, yielding proBNP. As proBNP is transported through the Golgi apparatus, glycosylation occurs at seven sites in the N-terminal region, and within the trans-Golgi network some of the proBNP is cleaved by furin into BNP and NT-proBNP (a portion remains as proBNP). Then, BNP, NT-proBNP, and proBNP are released into the blood. Circulating BNP binds to its receptors on various tissues and organs throughout the body (blood vessels, kidneys, adrenal glands, fat cells, etc.), after which it is internalized by the cell and metabolized. Circulating BNP is also degraded in the blood by proteases (neprilysin and others), and it is also filtered by the glomerulus, metabolized, and excreted in the urine. Binding of circulating proBNP to its receptor is weak, is not degraded by neprilysin and is less filtrated from the glomerulus. On the other hand, because circulating NT-proBNP does not bind to BNP receptors, nor is it degraded by enzymes such as neprilysin, its clearance depends almost exclusively on excretion via the kidney. NT-proBNP levels therefore increase sharply with even mild renal dysfunction and are markedly increased in renal failure.