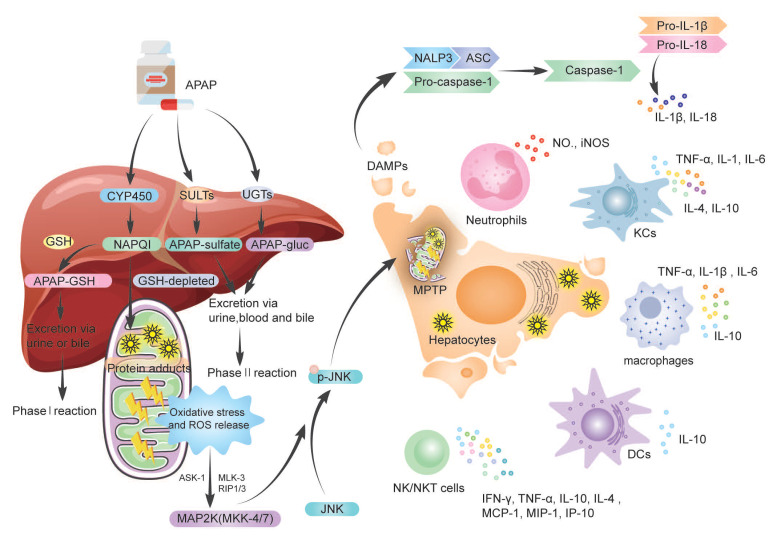
Figure 1.
The metabolism of APAP and the role of innate immune response in AILI. Following oral treatment, APAP is absorbed from the gut and transported to the liver for metabolism. A large portion (80–90%) of APAP is metabolized by SULTs and UGTs. A minor (5–10%) amount of APAP was metabolized in hepatocytes by CYP450 enzymes to the reactive metabolite NAPQI. The GSH rapidly converts NAPQI forming the APAP-GSH complex. When GSH is depleted, the growing concentration of NAPQI forms harmful APAP protein adducts, resulting in oxidative stress and increased ROS production in the mitochondria of hepatocytes. Protein adducts induce phosphorylation of JNK via ASK-1, MLK-3, and RIP1/3, then induce the MPTP opening, ultimately leading to hepatocyte necrosis and liver failure. The necrotic hepatocyte released various endogenous DAMPs, upregulating the infiltration of neutrophils, monocytes/macrophages, activated KCs, DCs, NK/NKT cells, and secreted cytokines such as TNF-α, IL-1, IL-6, IL-10, etc. DAMPs also activate inflammasomes, which participate in the innate immune response by activating caspase-1 to cleave pro-ILβ, and pro-IL-18 into IL-1β and IL-18. APAP—acetyl-para-aminophenol; AILI—APAP-induced liver injury; SULTs—sulfate transferase; UGTs—UDP glucuronosyltransferase; NAPQI—N-acetyl-p-benzoquinone imine; ROS—reactive oxygen species; MAPK—mitogen-activated protein kinase; JNK—c-Jun N-terminal kinase; MPTP—mitochondrial membrane permeability transition pore; DAMPs—damage-associated molecular patterns; KCs—Kupffer cells; DCs—Dendritic cells; NK/NKT cells—natural killer cells/NKT cells; TNF-α—tumor necrosis factor-α; IL—interleukin; INF—interferon; MCP—monocyte chemoattractant protein; MIP—macrophage inflammatory protein; IP—interferon-inducible protein; GSH—glutathione; CYP450—cytochrome P450; MLK-3—maxed-lineage kinase-3; ASK-1—apoptosis signal-regulating kinase-1; MKK—MAPK kinases; RIP1/3—receptor-interacting protein-1/3.