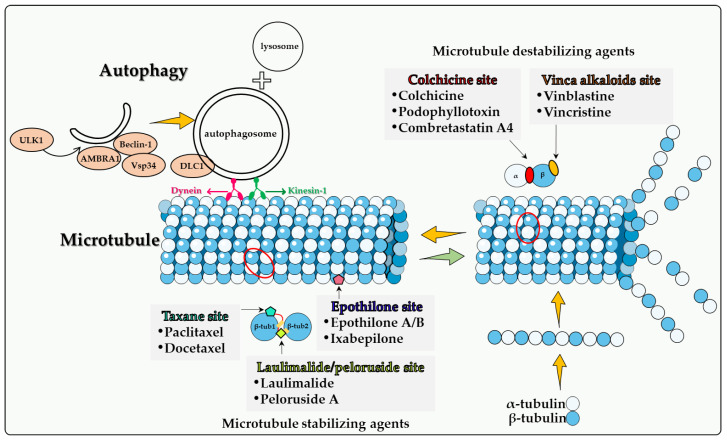
Figure 1.
The binding sites of microtubule poisons and autophagy. Kinesin-1 and dynein are microtubule motors that transport intracellular cargos. Once formed, the autophagosome travels along the microtubule via kinesin motor complexes, accumulating at the microtubule-organizing center, and eventually moving towards the lysosome. This centripetal movement is dependent on the motor protein dynein and is required for their fusion with lysosomes. Microtubule poisons are classified into two main categories, depending on whether they act as microtubule destabilizing agents or microtubule stabilizing agents. Microtubule destabilizing agents: Colchicine, Podophyllotoxins and Combretastatin A-4 target the colchicine site between α- and β-tubulin subunits (red dot); Vinca alkaloids bind to the β-tubulin subunit of the α/β-tubulin heterodimer (yellow dot). Microtubule stabilizing agents: Paclitaxel and Docetaxel target the Taxane site, binding to the β-tubulin subunit (green pentagon). Laulimalide and Peloruside A binding involves the bridging of two adjacent tubulin dimers (β-tubulin1 and β-tubulin2) across protofilaments in microtubules (light green squares). Epothilone sites and Taxane sites are different. Among the five oxygen-containing polar groups constituting the macrocycle of epothilone, only C7-OH is located near the similar C7-OH moiety of paclitaxel, and the polymerization activity of epothilone B is 2 to 10 times higher than that of paclitaxel (pink pentagon).