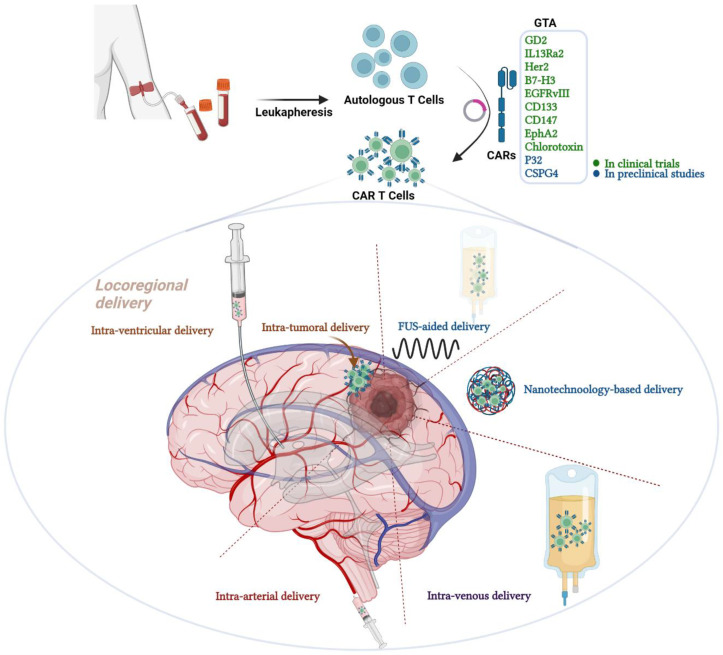
Figure 1.
Graphical abstract representing the different routes of chimeric antigen receptor (CAR) T cell therapy administration in malignant gliomas. CAR T cell therapy is one of the immunotherapeutic modalities under the umbrella of adoptive T cell therapy. CAR T cells are genetically modified autologous T cells that carry CARs to better recognize and attack cancer cells. The construct composed of antibody-derived extracellular ligand-binding domain, a hinging transmembrane domain and an intracellular T cell receptor (TCR)-derived signaling domain fortified with other co-stimulatory domains. Examples of GTAs that are targeted in clinical trials are GD2, IL13Ra2, HER2, B7-H3, EGFRvIII, EphA2 and chlorotoxin. Other GTAs that are under preclinical investigations include P32 and CSPG4. Several routes of administration are available for CAR T cell therapy but vary in the efficacy and safety; including locoregional delivery, FUS-aided delivery, nanotechnology-based delivery, intravenous and intra-arterial delivery. GTA; glioma target antigens, CARs; chimeric antigen receptors, GD2; disialoganglioside 2, IL13Ra2; interleukin 13 receptor subunit alpha 2, HER2; Human epidermal growth factor receptor 2, B7-H3; B7 Homolog 3, EGFRvIII; epidermal growth factor receptor variant III, EphA2; Ephrin type-A receptor 2 and FUS; Focused Ultrasound. Created with BioRender.com.