Figure 1.
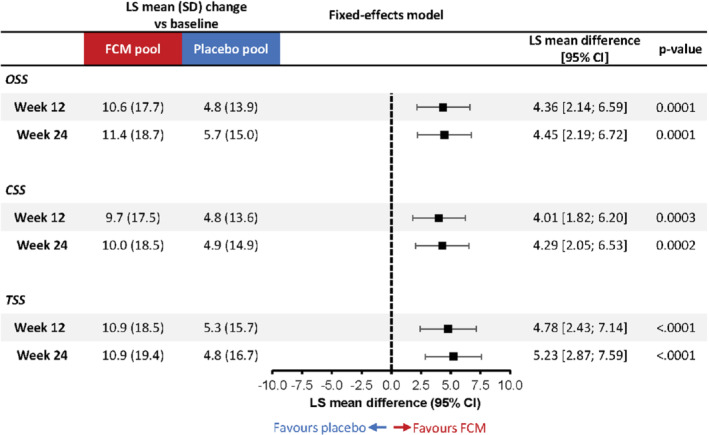
Mean change from baseline in the Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score (OSS), clinical summary sore (CSS) and total symptom score (TSS) with ferric carboxymaltose (FCM) versus placebo at weeks 12 and 24 (fixed‐effects model). Fixed‐effects mixed‐model for repeated measures analysis adjusted for study, baseline KCCQ score, age, estimated glomerular filtration rate, diabetes status, sex and left ventricular ejection fraction. CI, confidence interval; LS, least‐square; SD, standard deviation.
