Abstract
Background
Central neuropathic pain (CNP) is an excruciating condition, prevalent in up to a third of patients with multiple sclerosis (MS). Identifying CNP among MS patients is particularly challenging considering the ample comorbid chronic pain conditions and sensory disturbances entailed by the disease. The aim was to identify sensory features unique to CNP beyond those of chronic pain and MS.
Methods
Participants were 112 MS patients: 44 with a diagnosis of CNP, 28 with a diagnosis of chronic musculoskeletal pain (MSP), and 40 pain free. Participants underwent testing of thermal and mechanical thresholds, thermal grill illusion (TGI), pain adaptation (PA), and offset analgesia (OA), and chronic pain was characterized. A two‐step cluster analysis was performed, and the association between the cluster membership and the clinical group membership (CNP, MSP, pain free) was evaluated.
Results
The CNP and MSP groups were similar in most of the chronic pain variables (e.g., severity, location and quality) and MS‐related variables (e.g., type, severity and medication intake). The three created clusters had unique sensory features: (1) ‘Hyposensitivity’ (increased thermal and touch thresholds) characterized the CNP group; (2) ‘Poor inhibition and hyperalgesia’ (worst PA and OA and decreased TGI threshold) characterized the MSP group; and (3) ‘Efficient inhibition’ (best PA and OA, smallest sensory loss) characterized the pain‐free group.
Conclusions
The unique sensory features of CNP and MSP provide insight into their pathophysiology, and evaluating them may increase the ability to provide individually based interventions. Efficient inhibition may protect MS patients from chronic pain.
Significance
Cluster analysis among patients with multiple sclerosis (MS) revealed that while central neuropathic pain is associated with thermal and mechanical hypoesthesia, musculoskeletal pain is involved with reduced pain inhibition and hyperalgesia; sensory profiles that provide insights into the mechanisms of these conditions and may promote an individually based pain management.
1. INTRODUCTION
Multiple sclerosis (MS) and chronic pain are highly comorbid (Boneschi et al., 2008; Ferraro et al., 2018). The more common types of pain are spasticity pain and headache (prevalence rates of 43%–60%), central neuropathic pain (CNP, 5%–28%), and back pain (10%–20%) (Foley et al., 2013; Heitmann et al., 2020; O’Connor et al., 2008; Truini et al., 2013). CNP is considered highly excruciating and debilitating as well as particularly difficult to manage compared with other pain types (Doth et al., 2010; Österberg et al., 2005). The lifelong suffering imposed by CNP limits the patients’ functional capacity and quality of life (Doth et al., 2010; Failde et al., 2018).
The management of CNP in MS is challenged by the difficulty in its identification and hence in providing effective treatment. MS alone produces ample and widespread sensory alterations which may mask those related to CNP (Grasso et al., 2008; Pompa et al., 2015; Scherder et al., 2018). Furthermore, widespread pain, often of musculoskeletal origin (due to disuse/overuse injuries, abnormal posture, assistive technology usage, and decreased mobility) (Truini et al., 2013) may further complicate the distinction of CNP from other pain types (Feketová et al., 2017; Kahraman et al., 2019). As quantitative sensory testing (QST) is eminent in characterizing pain conditions and studying their mechanisms (Arendt‐Nielsen & Yarnitsky, 2009; Backonja et al., 2013), a systematic comparison of QST results from MS patients with CNP vs. those with musculoskeletal pain (MSP) may help to discern the unique features of each condition and shed more light on their pathophysiology.
We found only one study in which such a comparison has been conducted: Thermal and touch thresholds were similar among 29 MS patients with CNP and 15 MS patients with MSP (Svendsen et al., 2005). The lack of a pain‐free control group and the since‐updated CNP diagnostic criteria, necessitates revisiting this comparison with additional tests. The QST of MS patients with CNP has been compared with that of pain‐free MS patients. Overall, a significant reduction in thermal sensibility was reported among the former, as well as an increased frequency of allodynia and hyperpathia (Österberg & Boivie, 2010; Rivel et al., 2021; Srotova et al., 2020). However, considering that sensory impairment is an integral sign of MS and may occur regardless of chronic pain, it is not clear whether these features are unique to CNP or characterize all MS‐related chronic pain types. Indeed, MS patients with chronic pain of unspecified origins had thermal and pinprick thresholds similar to those of pain‐free MS patients (Fernández‐de‐las‐Peñas et al., 2015; Grasso et al., 2008; Pompa et al., 2015).
Thus, because QST of MS patients with CNP has been compared with that of a single control group, it is unclear whether CNP in MS has distinct features beyond those of chronic pain in general or of MS; such features may promote knowledge about its mechanisms. Furthermore, despite the known association of various chronic pain types with reduced pain modulation (Arendt‐Nielsen et al., 2018), only one study explored this association among CNP‐ and pain‐free MS patients (Srotova et al., 2020). The current study’s aims were therefore to: (1) characterize the sensory and pain modulation profile of MS patients with CNP compared with patients with MSP and pain‐free MS patients, and (2) perform a cluster analysis in order to identify distinctive characteristics of CNP beyond those of other chronic pain and MS.
2. METHODS
2.1. Participants
Participants were 112 MS patients in three groups according to clinical diagnosis: 44 MS patients had CNP (the CNP group); 28 MS patients had musculoskeletal pain (the MSP group) and 40 MS patients were pain‐free (the pain‐free group). The MS patients were recruited from the outpatient clinic in the Multiple Sclerosis Center at Sheba Medical Center, Tel Hashomer, between the years 2015 and 2018. Only patients with definite MS were recruited. The diagnosis of definite MS was determined by a neurologist, according to the revised McDonald’s criteria (Polman et al., 2010). Inclusion criteria for all MS patients were as follows: (1) diagnosis of MS, (2) disease duration of at least 1 year, and (3) Age =>18 years, <=75 years (the latter due to possible changes in sensory sensitivity). MS patients in the pain‐free MS group and healthy controls had to be free of any acute or chronic pain. Exclusion criteria for all the participants were as follows: (1) pregnancy, (2) systemic diseases (e.g. diabetes) or concurrent neurological conditions, (3) skin lesions at the testing sites, (4) psychiatric diseases (e.g. major depression) or any other cognitive condition that might interfere with testing and/or completing the questionnaires. It should be noted that disability status of the patients or other MS‐related complaints were not considered for recruitment in order to avoid selection biases.
Eligible participants were approached by the medical staff of the unit and received information on the study, both orally and by way of flyers. Those individuals who agreed to participate in the study contacted the phone number on the flyers or informed members of the medical staff. After a short screening interview, they were invited to a meeting in order to ascertain their eligibility. For the chronic pain groups, MS patients with pain lasting more than 6 months were included, provided that the onset of the pain was subsequent to an established diagnosis of MS (O’Connor et al., 2008; Truini et al., 2013). The diagnosis of CNP and MSP was made by a pain specialist. The presence of CNP was determined according to its definition and characteristics with specific relevance to MS (Widerström‐Noga et al., 2017) as follows: (1) a diagnostic evaluation confirming MS, (2) continuous or recurrent pain developed after MS onset, (3) pain duration of at least 3 months, (4) pain is described as being within the area of the body affected by an MS lesion in the brain or spinal cord, (5) pain is associated with sensory changes in the same neuroanatomically plausible distribution, as indicated by the presence of at least one positive sensory sign (e.g., dynamic mechanical or cold allodynia) or one negative sensory sign (e.g., elevated thresholds to cold or warm sensations or a decreased sensation to touch, a pinprick, or thermal stimuli), and (6) no other diagnosis better explains the pain. The presence of MSP was determined according to its characteristics (Truini et al., 2013): (1) pain is associated with muscle and/or skeletal system and derives from an abnormal posture or decreased mobility, (2) pain is not related to peripheral or central nervous lesions, (3) no other diagnosis better explains the pain.
The study was approved by the institutional review board of Sheba Medical Center and of Tel Aviv University. Written informed consent was obtained from all participants, after they received a full explanation of the study’s protocol and goals.
2.2. Procedures
The sample size was estimated a priori as indicated in our previous publication (Rivel et al., 2021). In short, we considered expected means and standard deviations of two main outcome measures (warm sensation threshold and pain adaptation), α = 0.05, and a statistical power of 80% (effect size ranged between 0.85 and 0.92). The calculation yielded a sample size of 14–19 participants per group but we made efforts to double the sample size due to the expected variability in these measures among the chronic pain groups.
Testing took place in a quiet room. Each participant arrived to a single testing session in which she/he was interviewed regarding demographics, general health status, and MS‐ and pain‐related variables and completed two questionnaires. Sensory testing then commenced following a training session. Testing included: (1) the measurement of warm sensation threshold and cold sensation threshold, as indicators of spinothalamic conduction (Willis & Westlund, 1997); (2) mechanical detection threshold, as an indicator of the dorsal column/medial lemniscal conduction (Noback et al., 2012); (3) the thermal grill illusion, as an indicator of central integration of thermal pathways and pain (Craig & Bushnell, 1994; Jutzeler et al., 2017); and (4) pain adaptation and offset analgesia, as indicators of central pain inhibition (D’Agata et al., 2015; Shulman et al., 2020). Testing was done in a random order. Participants were given a 2–5‐min break between tests, and the probe of the stimulator was moved after each stimulation to an adjacent region in order to prevent changes in skin sensitivity due to stimulation. The first three aforementioned sensory tests were performed in the most painful body region: the shin among 80% of the CNP group and among 70% of the MSP group, and the arm/forearm among 20% and 30% of these groups, respectively. Data obtained from the most painful lower limb sites and the most painful upper limb sites were combined. Among the pain‐free group, testing was performed at the shin, which was the most prevalent painful region among both chronic pain groups (testing of this group was conducted after the majority of the participants with chronic pain completed their testing). Note that the lower limbs in both the CNP and MSP groups were significantly more affected by sensory deficits than the upper limbs (please see supplementary table). Pain adaptation and offset analgesia tests were performed in the proximal volar aspect of the forearm which was pain free and relatively intact among the majority of the participants. The reason for choosing this location was that these tests necessitated a stimulus‐response function in order to search for the temperature that would elicit a particular level of pain. Such a test is more reliable in a relatively preserved body region. Among patients for whom this region was painful or exhibited significant sensory alteration, an adjacent region was selected.
2.3. Equipment
2.3.1. Thermal stimulator
Heat and cold stimuli were delivered using the conditioned pain modulation (CPM) system (Q‐Sense‐CPM, Medoc, Ltd., Ramat Yishai, Israel). The computerized Medoc Q‐Sense‐CPM system has two thermods with an active area of 30 × 30 mm and a temperature range from 20°C to a safety limit of 50°C. Heating or cooling is produced using a Peltier‐based computerized thermal stimulator. The adaptation (baseline) temperature was set to 32°C. The probe was attached to the testing site by means of a Velcro band. If the testing site was too large, the examiner held the probe gently over the tested skin and verified that the probe surface made full contact.
2.3.2. Semmes–Weinstein monofilaments
Mechanical stimuli were applied with Semmes–Weinstein monofilaments (North Coast Medical, Inc., Morgan Hill, CA, USA). The kit consists of 20 monofilaments with sizes ranging between 1.65 and 6.65 units, each attached to a plastic holder. Vertical bending of the monofilament produces a calibrated force ranging between 0.008 and 300 grf.
2.4. Sensory testing
2.4.1. Thermal sensibility
Warm sensation threshold (WST) and cold sensation threshold (CST) were measured with the computerized thermal stimulator, using the method of limits. For each threshold separately, participants received four successive stimuli of gradually decreasing or increasing temperatures, starting from a baseline of 32°C, at a rate of 2°C/s. The participants were asked to press a switch (the computer mouse) when a cold or warm sensation, respectively, was first perceived. Each threshold was calculated as the average of four successive temperatures in each test (for more details, please see Rivel et al., 2021). The complete absence of an innocuous thermal sensation was defined when the participant did not perceive warming or cooling up to the cut‐off values of 43°C or 20°C, respectively. These cut‐off values were determined according to (1) the average warm and cold sensation thresholds of healthy controls ±3 standard deviations from the mean, and (2) the upper temperature response limit of warm and cold receptors, which is below the lower temperature response limit of nociceptors, as reported elsewhere (Dodt & Zotterman, 1952; Iggo, 1969).
2.4.2. Mechanical sensibility
Mechanical detection threshold (MDT) was tested using the modified method of limits. While participants were blindfolded, the examiner applied the Semmes–Weinstein monofilaments in an increasing order, starting from the smallest one (maximal range 0.064–1.143 mm). The participants were asked to respond the minute they perceived a touch, at which point they were asked to localize the stimulus perceived. MDT was the calibrated force of the monofilament first perceived (Zeilig et al., 2012). There was no need for cut‐off values in these tests because all the participants perceived touch.
2.4.3. The Thermal Grill Illusion (TGI)
The TGI was evoked with the computerized thermal stimulator, to which two probes were connected. The probes were placed adjacent to each other. One probe was set for cooling, and the other was set for warming. Both probes were activated simultaneously at a rate of 2°C/s starting from an adaptation temperature of 32°C, and the participants were instructed to press the switch when they perceived the first pain sensation, thus defining the TGI threshold. At that moment, the temperatures of both the warming and cooling probes were recorded and stopped, and participants were asked to indicate the quality of pain (from a list of descriptors: burning pain, cold pain, mixed burning/cold, and pricking pain) (Defrin et al., 2008). The difference between the temperatures measured in each probe indicated the TGI level. Thus, the smaller the difference, the more sensitive the person was to the TGI.
2.4.4. Pain inhibition
As pain inhibition has hardly been tested among patients with MS, we used two pain inhibition tests: pain adaptation (PA) and offset analgesia (OA). PA refers to a gradual decrease in perceived pain following repeated/constant, moderate noxious stimuli of fixed intensity (Bauch et al., 2017; Bingel et al., 2007). OA refers to a sharp decrease in perceived pain following an increase and then a decrease in the same magnitude, of a noxious stimulus relative to initial intensity (Derbyshire & Osborn, 2008; Grill & Coghil, 2002).
In order to test PA level, participants received a noxious heat stimulus (using the thermal stimulator) at an intensity equivalent to 5 out of 10, on the numerical rating scale (NRS). The stimulus was applied for 30 s during which time the participants were asked to rate the amount of perceived pain (using the NRS) every 15 s (at times 0, 15 and 30 s). The magnitude of PA was calculated by subtracting the first NRS rating from the last (Gruener et al., 2016). The participants were not informed of the time that had elapsed from the beginning of stimulation.
To test for OA, participants received a noxious heat stimulus (using the thermal stimulator) at an intensity equivalent of 6 out of 10 on the NRS for a duration of 5 s, which then increased by 1°C for a duration of an additional 5 s and afterwards decreased by 1°C to the initial intensity (5–6 on the NRS) and remained at that intensity for 20 additional seconds. The participants were asked to rate the amount of perceived pain (using the NRS) at six time points that capture the different phases of the stimulus: at the peak of the initial intensity (Time 1); at the peak of the 1°C increase (Time 2); immediately after the 1°C decrease (Time 3) and three consecutive times during the plateau, every 5 s (Time 4–6 the last rating just near the end of the plateau). The magnitude of OA was the difference between T1and T3 (Grill & Coghil, 2002). The temperatures that were chosen for the pain adaptation and OA tests were extracted from individual stimulus‐response functions for heat‐pain created for each participant, in which the participants rated a series of thermal stimuli (ranging from 40°C to 50°C) using the NRS (for more details, please see Gruener et al., 2016).
2.5. Additional data collection
Data on patients’ MS (e.g., Expanded Disability Status Scale/EDSS, which quantifies disability in MS, duration, first symptoms appearance and diagnosis, MS type, motor and sensory dysfunctions, and course) and general health status were obtained via a structured interview and from the patients’ medical records. Data on chronic pain were obtained via a structured interview that included questions about pain onset, duration, quality, location in the body (on a body chart), dynamic characteristics, use of medications, as well as alleviating and aggravating factors. The number of painful body regions was counted from the body chart, divided into 13 regions: foot, shin, thigh, buttocks, hand, forearm, arm, shoulder, abdomen, chest, lower back, upper back, neck. Participants with chronic pain also rated the mean chronic pain intensity during the previous month using the NRS and completed the McGill Pain Questionnaire (MPQ) (Melzack, 1975), which provides a quantitative evaluation of the participants’ pain experience as follows: the pain rating index (PRI) – the total of the values assigned to the words chosen from a list of 64 pain descriptors; the number of words chosen (NWC) from that list; Pleast – the lowest pain intensity (on a Likert‐type scale from 1 to 5); and Pworst – the highest pain intensity (on a scale of 1 to 5). In addition, the Douleur Neuropathique 4 (DN4 questionnaire) (Bouhassira et al., 2005) was completed.
2.6. Statistical analysis
Data were processed with IBM SPSS statistics software version 27 (IBM New York, USA). First, normal distribution was evaluated with the Kolmogorov–Smirnov (K‐S) test. Parametric and non‐parametric models with interactions and post hoc corrected comparisons were used in order to test the effect of group type (CNP group, MSP group, pain‐free group) on demographics (age, sex, education and employment), MS‐related data (MS duration, EDSS and MS course/type) and sensory testing (WST, CST, TGI, MDT, PA and OA). As there was a group difference in EDSS, MS duration and in age, and considering the possible effect of these variables on the sensory testing, they were entered as covariates in the models. Data are presented as mean ±standard deviation (SD) for continuous variables and as count (percentage) for categorical variables. All tests were two‐tailed, and statistical significance was defined as a value of p ≤ 0.05.
In order to examine whether the CNP group exhibited unique features as compared with the rest of the MS cohort, a two‐step cluster procedure was conducted. Instead of predicting an outcome, two‐step cluster models uncover patterns in a data set and classify the entire data set into natural groups (clusters) that would otherwise not be apparent. Thus, records within a group or cluster tend to be similar to each other, but records in different groups are dissimilar. The two‐step cluster analysis was chosen for classification because it can handle mixed‐field types and is able to handle large datasets efficiently. It also provides the importance of predictor variables included in the analysis (Gelbard et al., 2007). The classification is established according to the data (in the present case, the results of the sensory tests), irrespective of previously known groups/diagnoses or outcome measures, while the strength of the contribution of each sensory test to the classification (predictor importance) is taken into account. The first step makes a single pass through the data, during which it compresses the raw input data into a manageable set of subclusters. The second step uses a hierarchical clustering method to progressively merge the subclusters into larger and larger clusters, without requiring another pass through the data. The number of clusters was pre‐determined to three as we were interested in examining the associations between natural groups (clusters) defined by the clustering procedure and the three clinically diagnosed groups. The model’s fit was assessed by Schwarz’s Bayesian information criterion (BIC) and evaluated by the average silhouette coefficient, which evaluates cluster cohesion and separation measures (Kaufman & Rousseeuw, 2005).
Following the two‐step clustering procedure in which individuals were assigned a cluster membership, a crosstabs analysis was performed in order to test the association (χ2) and the strength of the association (Cramer’s V‐ rc) between the cluster membership and the group membership (CNP, MSP, pain free). Multivariate ANOVA (MANOVA) as well as χ2 analyses were performed in order to detect differences between the clusters in demographics and in MS‐related variables. In order to compare QST data within and between clusters, and following the approach of Baron et al. (2017), the results were z‐transformed, based on sensory testing done among 34 healthy participants using the same methods and by the same examiner (data on this group are detailed in Rivel et al., 2021. In short, the sample comprised 27 healthy females and seven healthy males, at a mean age of 43.68 ± 11.25 and mean education years of 16.24 ± 2.49, free of any acute or chronic pain, and in good general health). The data are presented ±95% confidence intervals. Z scores of ‘0’ represent the mean value of the healthy participants, whereas z scores above or below ‘0’ indicate a gain or loss of function, respectively, for sensory thresholds, and efficient or inefficient pain inhibition, respectively, for OA and pain adaptation.
3. RESULTS
3.1. Characteristics of the study groups
Table 1 describes the characteristics of the three MS groups: CNP, MSP and pain free. The two chronic pain groups (CNP and MSP) did not differ in sociodemographic data, although the MSP group was significantly older than the pain‐free group. A significantly smaller percentage of patients in both chronic pain groups were employed, compared with the percentage in the pain‐free group. The two chronic pain groups did not differ in MS‐related variables except for the duration of the disease, which was significantly longer among the MSP group, and both groups had a significantly higher EDSS score than did the pain‐free group. The etiologies among patients with MSP were spasticity‐related pain (n = 16), non‐radicular back pain (n = 7), articular pain (n = 8), myofascial pain (n = 4), fibromyalgia (n = 1) and seven people had dual etiologies. Pain medication intake was similar for the two chronic pain groups except for NSAIDs, which were used by a greater proportion in the CNP than the MSP group. The NSAID medications included paracetamol, dipyrone, and ibuprofen.
TABLE 1.
Characteristics of the study groups
| MS patients with CNP | MS patients with MSP | MS patients pain free | ANOVA# | |
|---|---|---|---|---|
| Participants (number) | 44 | 28 | 40 | |
| Age (years, m±SD) | 42.7 (10.7) | 48.0 (12.0)3** | 39.3 (12.2) | <0.05 |
| Sex (females, %) | 29 (64.4) | 21 (75.0) | 24 (57.1) | 0.31 |
| Employment (yes, %) | 18 (40.9)2* | 14 (50.0)3** | 34 (81.0) | <0.01 |
| Education (years, m±SD) | 14.2 (2.3) | 14.4 (3.7) | 15.0 (2.5) | 0.40 |
| MS (years, m±SD) | 9.6 (6.5)1** | 16.6 (9.0)3** | 10.9 (6.4) | <0.01 |
| EDSS (m±SD) | 4.3 (2.0)2** | 5.0 (1.7)3** | 3.1 (1.7) | <0.01 |
| MS Type (RR, %)^ | 39 (88.6) | 19 (67.9)3** | 39 (97.5) | < 0.01 |
| IMiDs (yes, %) | 38 (86.4) | 24 (88.9) | 32 (80.0) | 0.62 |
| Steroids (yes, %) | 14 (31.8) | 6 (22.2) | 18 (45.0) | 0.12 |
| Pain medications | ||||
| NSAIDs (yes, %) | 14 (29.8)2** | 4 (14.8) | 1 (2.4) | <0.01 |
| Antiepileptics (yes, %) | 11 (25.0) | 5 (18.5) | ─ | 0.49 |
| Medical marijuana (yes, %) | 8 (18.1) | 3 (11.1) | ─ | 0.37 |
| Opioids (yes, %) | 6 (13.6) | 1 (3.7) | ─ | 0.14 |
| SNRI (yes, %) | 6 (13.6) | 2 (7.4) | ─ | 0.49 |
MS=Multiple Sclerosis, CNP=central neuropathic pain, MSP=musculoskeletal pain, m=mean, SD=Standard Deviation, EDSS=Expanded Disability Status Scale, RR=Relapsing Remitting (^the rest are secondary progressive MS), IMiDs=Immunomodulatory drugs, NSAIDs=Non‐steroidal anti‐inflammatory drugs, SNRI=Serotonin/noradrenaline reuptake inhibitors, #p‐values of ANOVA effects (parametric and non‐parametric tests). Asterisks are post hoc corrected two‐tailed t‐tests (for parametric variables) or corrected two‐tailed Mann–Whitney tests (for non‐parametric variables) between each two groups: 1= MS patients with central neuropathic pain vs. MS patients with musculoskeletal pain, 2= MS patients with central neuropathic pain vs. MS patients without pain, 3= MS patients with musculoskeletal pain vs. MS patients without pain (*p < 0.05, **p < 0.01).
3.2. Chronic pain characteristics
Table 2 presents data on the chronic pain characteristics among MS patients with CNP and those with MSP. Chronic pain severity on average, as well as chronic pain severity at its worst, were similar in magnitude for the two groups, although the indices of the MPQ showed a trend towards being higher in the CNP group. The level of pain interference and the duration of chronic pain were also similar for the two groups. The only significant difference between the two chronic pain groups was in the number of painful body regions: the CNP group exhibited a greater number than did the MSP group, suggesting more widespread pain among the former. Generally, in both groups, chronic pain was located especially in the legs. The most frequent painful region among the CNP group was the shin (39 patients/88.6%), and among the MSP group, the shin and thigh (15/53.6% each, p < 0.01 compared to CNP). Patients of both groups also had headaches: The prevalence of headaches in the CNP group was 33.3% (15/45), and in the MSP group it was 7% (2/28, p < 0.05). The quality of CNP was described as heat or burning by the majority of the CNP group (28/63.6%), followed by sharp (24/54.5%) and radiating (19/43.2%). Patients with MSP described the pain mostly as sharp (15/53.6%) but also as radiating (12/46.4%) and burning (10/35.7%, p < 0.05 compared with CNP).
TABLE 2.
Chronic pain characteristics among the CNP and MSP groups
| MS patients with CNP | MS patients with MSP | p‐value | |
|---|---|---|---|
| NRS of average pain (0–10) | 7.22 (1.89) | 6.68 (1.98) | 0.25 |
| PRI total (0–64) | 27.15 (11.61) | 21.74 (9.76) | 0.08 |
| PRI sensory | 8.03 (3.06) | 6.48 (2.95) | |
| PRI affective | 2.41 (1.25) | 2.04 (1.58) | |
| PRI evaluative | 1.46 (0.55) | 1.34 (0.57) | |
| Worst pain severity (1–5) | 4.38 (0.88) | 4.22 (0.74) | 0.23 |
| Number of painful regions (0–22) | 8.07 (4.16) | 3.29 (2.19) | <0.01 |
| Pain interference (0–30) | 17.13 (7.79) | 15.80 (7.40) | 0.50 |
| Pain duration (years) | 6.20 (5.36) | 6.35 (6.63) | 0.92 |
| DN4 questionnaire (0–10) | 5.39 (1.38) | 1.39 (1.73) | 0.00 |
MS=Multiple Sclerosis, CNP=Central neuropathic pain, MSP=Musculoskeletal pain, SD=Standard Deviation, NRS=Numerical rating scale, PRI=Pain rating index from the McGill pain questionnaire, NWC=Number of words chosen from the McGill pain questionnaire, p‐values of corrected two‐tailed t‐tests or Mann–Whitney U test (for worst pain severity).
With regard to allodynia assessed during physical examination, mechanical allodynia was evoked in about a third of the CNP group (12/27% participants) and in about a fifth of the MSP group (6/21.4%) (p = 0.47). Cold allodynia was evoked in a third of the CNP group (12/27% participants) and in about 14% of the MSP group (4/28) (p = 0.21). In total, 21/45 of the CNP group had either mechanical or cold allodynia or both allodynia types, and 7/28 had it/them in the MSP group (p = 0.06).
3.3. Comparison between groups in sensory testing
3.3.1. Thermal and mechanical sensibility
Analysis of covariance (ANCOVA) revealed a significant effect of group type on warm, F(2,112) = 5.503, p < 0.01, and cold thresholds, F(2,112) = 13.085, p < 0.0001. Post hoc comparisons revealed that the CNP group had significantly higher warm and cold thresholds compared with both the MSP (p < 0.01 and p < 0.01, respectively) and pain‐free groups (p < 0.05 and p < 0.01, respectively) (Figure 1a and b, respectively). With regard to touch threshold, ANCOVA revealed no significant differences between the groups, F(2,112) = 0.175, p = 0.840 (Figure 1c).
FIGURE 1.
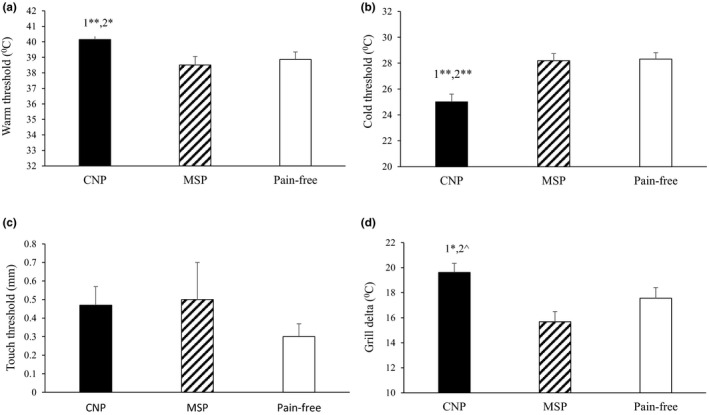
Warm (a) and cold (b) thresholds as well as the thermal grill delta (d) were increased among the CNP group compared with the MSP and pain‐free groups. There were no group differences in touch threshold (c). Corrected pairwise two‐tailed group comparisons; 1= CNP vs. MSP, 2= CNP vs. pain free (^p = 0.057, *p < 0.05, **p < 0.01). Bars denote group mean ±SE. CNP=central neuropathic pain, MSP=musculoskeletal pain
3.3.2. Thermal grill illusion
Figure 1d presents the differences in TGI level between the groups. ANCOVA revealed a significant effect of group type on TGI level, F(2,112) = 5.880, p < 0.01. Post hoc comparisons revealed that the CNP group had a significantly higher TGI level compared with the MSP group (p < 0.01) and a trend towards a higher TGI compared with the pain‐free group (p = 0.058).
3.3.3. Pain inhibition
Figure 2 presents the two pain inhibition tests. ANCOVA revealed a significant effect of group type on pain adaptation, F(2,112) = 3.153, p < 0.05. Post hoc comparisons revealed that the CNP group had a significantly decreased pain adaptation compared with the pain‐free group (p < 0.01); however, no significant differences were found between the CNP and MSP groups (p = 0.214) (Figure 2a). With regard to OA, ANCOVA revealed no significant group effect, F(2,112) = 0.645, p = 0.527) (Figure 2b). Chronic pain severity as measured with the MPQ correlated significantly with pain adaptation level only in the CNP group (PRI: r = 0.35, p=0.032; NWC: r = 0.33, p = 0.041); namely, the worse the pain severity, the worse the pain inhibition level (positive values for pain adaptation suggest lack of pain reduction). No such correlation was found for the MSP group nor for OA in the CNP or MSP groups.
FIGURE 2.
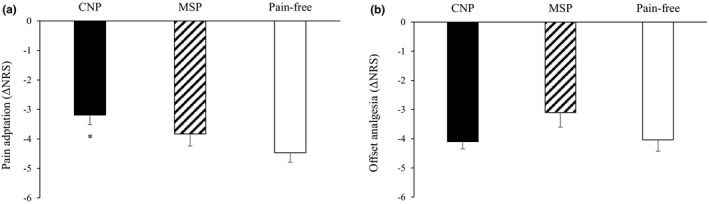
Pain adaptation was reduced among the CNP group compared with the pain‐free group (a; *p < 0.05). However, there were no group differences in offset analgesia (b). Bars denote group mean ±SE. CNP=central neuropathic pain, MSP=musculoskeletal pain, NRS=Numerical rating scale
3.4. Cluster analysis
The clusters analysis exhibited a fair cohesion and separation (average silhouette = 0.3) and a good ratio of sizes (ratio = 1.18) between the three clusters. Table 3 presents the QST predictors according to level of their prediction importance (in descending order) and the differences between the three clusters in the QST data using ANCOVA. Cluster 1 consisted of 40 patients and was characterized by the highest thermal and touch thresholds compared with Clusters 2 and 3, and a higher TGI threshold than Cluster 2. Thus, Cluster 1 was termed ‘hyposensitivity’. In contrast, Cluster 2 was characterized by the poorest level of pain inhibition compared with Clusters 1 and 3, and the lowest TGI threshold; hence it was termed ‘poor inhibition & hyperalgesia’. Cluster 3 had the best pain inhibition levels compared with both Clusters 1 and 2, and the lowest thermal and mechanical thresholds. Thus, this cluster was termed ‘efficient inhibition’. Table 3 also presents the demographic data and MS‐related factors among the three clusters. The only significant difference between the clusters was seen in age and EDSS score; the patients in Cluster 1 were about 7 years younger and had lower EDSS scores compared with the patients in both Clusters 2 and 3, who were similar. Nevertheless, ANCOVA revealed that neither EDSS nor age had a significant effect on the between‐cluster differences in the sensory testing.
TABLE 3.
Between‐cluster comparison of QST predictors (ordered according to prediction importance), sociodemographic and MS‐related factors
| Cluster 1 | Cluster 2 | Cluster 3 | ANOVA# | |
|---|---|---|---|---|
| Number | 37 (33%) | 35 (31.2%) | 40 (35.7%) | |
| QST predictors in descending order of importance | ||||
| Cold sensation (°C, m±SD) | 22.54 (3.05)1**,2** | 28.72 (1.63) | 29.53 (1.31) | <0.0001 |
| Offset analgesia (°C, m±SD) | −3.73 (1.89)1**,2** | −2.12 (1.27)3** | −5.67 (1.75) | <0.0001 |
| Warm sensation (°C, m±SD) | 42.37 (2.07)1**,2** | 38.20 (2.61) | 37.74 (2.56) | <0.0001 |
| Pain adaptation (°C, m±SD) | −3.13 (2.15)2** | −2.64 (1.77)3** | −5.45 (1.44) | <0.001 |
| Thermal grill (°C, m±SD) | 19.78 (5.22)1*** | 15.82 (4.05)3* | 18.18 (5.64) | <0.01 |
| Mechanical detection (mm, m±SD) | 0.67 (1.14)1*,2* | 0.33 (0.33) | 0.25 (0.35) | <0.01 |
| Demographics and MS‐related factors | ||||
| Age (years, m±SD) | 37.98 (9.68)1*,2** | 44.57 (13.84) | 45.73 (11.13) | <0.01 |
| Sex (females, %) | 26 (65) | 26 (74.3) | 19 (51.4) | 0.13 |
| Employment (yes, %) | 27 (67.5) | 20 (57.1) | 17 (45.9) | 0.16 |
| Education (years, m±SD) | 14.48 (3.44) | 14.83 (2.54) | 14.33 (2.17) | 0.74 |
| MS (years, m±SD) | 11.08 (6.83) | 12.25 (9.05) | 11.87 (7.41) | 0.80 |
| EDSS score (m±SD) | 3.21 (1.73)1*,2** | 4.13 (2.22) | 4.77 (1.62) | <0.01 |
| MS Type (RR, %)^ | 37 (92.5) | 29 (82.9) | 29 (78.4) | 0.21 |
MS=Multiple Sclerosis, m=mean, SD=mean ± standard deviation, EDSS=Expanded Disability Status Scale, RR=Relapsing Remitting (^the rest are secondary progressive MS). #p‐values of ANCOVA effects (EDSS, MS duration and age as covariates). Asterisks are post hoc corrected 2‐tailed t‐tests between each two clusters: 1= Cluster 1 vs. Cluster 2, 2= Cluster 1 vs. Cluster 3 (*p<0.05, **p<0.01, ***p<0.001).
Figure 3a presents the QST profiles of the three clusters as mean z scores ±95% confidence interval. Negative z values in the case of WST, CST, MDT and TGI represent loss of function, namely hypoesthesia and hypoalgesia, respectively, and in the case of PA and OA these represent less efficient inhibition. Positive z values in the case of WST, CST, MDT and TGI reflect gain of function, namely hyperesthesia and hyperalgesia, respectively, and in the case of PA and OA these represent more efficient pain inhibition. The dashed lines represent a 95% confidence interval for normative values (healthy participants −1.96 < z < +1.95), and the values of the MS groups are significantly different from those of healthy participants, if their 95% confidence interval does not cross the zero line.
FIGURE 3.
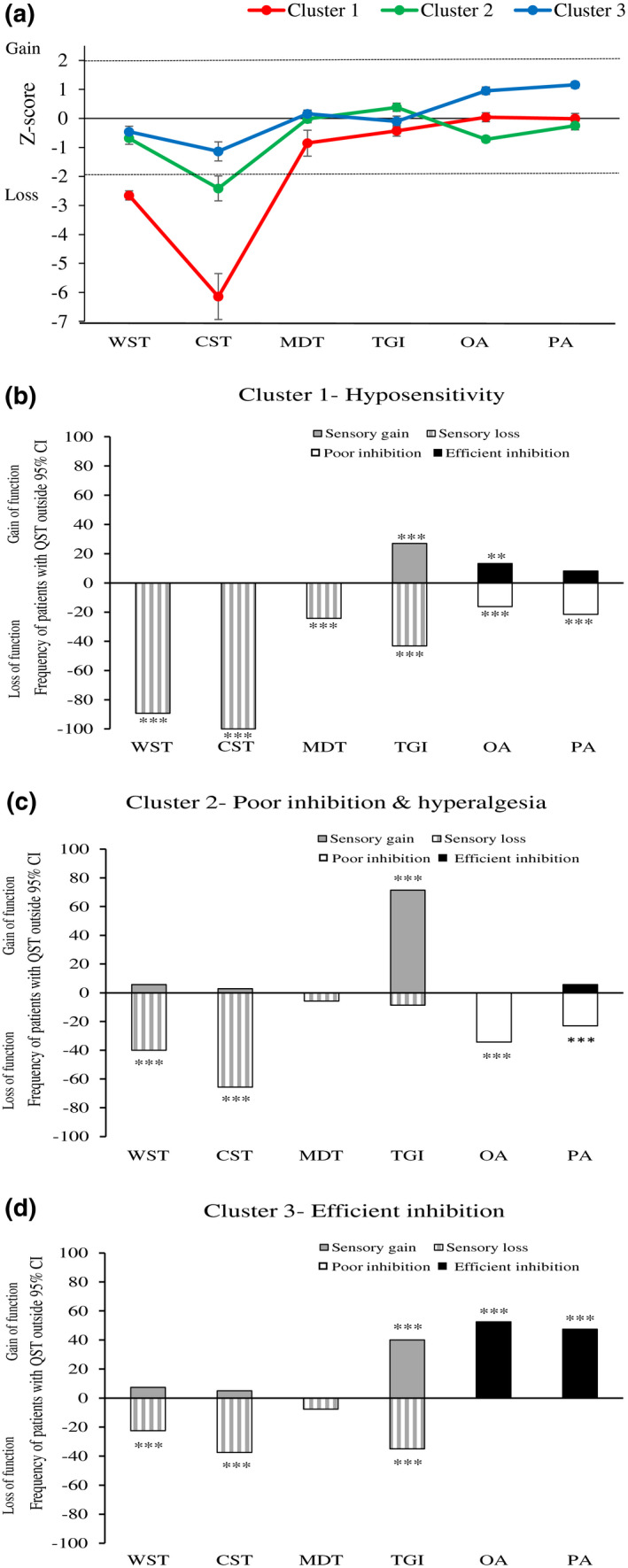
(a) Sensory profiles of the three clusters presented as mean z‐scores ±95% confidence interval: dashed lines represent 95% confidence interval for healthy participants (−1.96 < z < +1.96). Values are significantly different from those of healthy participants, if their 95% confidence interval does not cross the zero line. (b–d) Values are frequencies of patients with abnormal QST (beyond 95% CI of healthy participants) within Cluster 1(B), Cluster 2 (C) and Cluster 3 (D) (asterisks signify comparison to expected, normal values 2.5%; *p < 0.05, **p < 0.01, ***p < 0.001). For WST, CST, MDT, and TGI, negative bars indicate loss of sensibility (hypoesthesia, hypoalgesia), and positive bars indicate gain of sensibility (hyperesthesia, hyperalgesia). For OA and PA, negative bars indicate poor pain inhibition, and positive bars indicate efficient pain inhibition. WST=Warm sensation threshold, CST=Cold sensation threshold, MDT=Mechanical detection threshold, TGI=Thermal grill illusion, OA=Offset analgesia, PA=Pain adaptation
Thus, for innocuous thermal sensations (WST, CST), all the clusters exhibited decreased sensitivity, although Cluster 1 exhibited the greatest deficit, compared with that of Clusters 2 and 3, especially in CST, followed by Cluster 2. For touch sensation (MDT), Clusters 2 and 3 exhibited close to normal mean z‐scores, and Cluster 1 exhibited a slight decrease in sensitivity (mean z‐score = −0.852). For TGI level, Cluster 1 exhibited loss of function, namely hypoalgesia, whereas Cluster 2 exhibited a gain of function, namely hyperalgesia. Cluster 3 exhibited values close to zero. In the pain inhibition tests, Cluster 1 exhibited a mean z‐score close to zero, suggesting values close to those of healthy controls, whereas Cluster 3 exhibited a positive mean z‐score in both PA and OA, suggesting values of pain inhibition that were highly efficient (a comparison of PA and OA raw values of Cluster 3 with those of healthy controls resulted in significantly better values for the former group; p < 0.01 for both). Cluster 2 exhibited a negative mean z‐score, specifically in the OA test, reflecting poorer pain inhibition than normal values. Overall, Cluster 1 exhibited significant deficits in thermal sensitivity; Cluster 2 exhibited significant deficits in pain inhibition as well as hyperalgesia; and Cluster 3 exhibited the smallest sensory deficit and the best pain inhibition profile (Figure 3a).
Figure 3b‐d describes the percentage of patients in each cluster whose QST data were outside the 95% confidence interval after transforming the data into z‐scores. In Cluster 1, more than 80% of patients had reduced innocuous thermal sensitivity, and more than 40% had reduced sensitivity to the TGI (Figure 3b). In Cluster 2, between 40 and 60% of patients had reduced innocuous thermal sensitivity; however, more than 70% had an increased sensitivity to the TGI. Moreover, 20%–40% had reduced pain inhibition (Figure 3c). In Cluster 3, less than 40% had reduced innocuous thermal sensitivity, whereas more than 40% had more efficient pain inhibition (Figure 3d).
Table 4 presents the two‐step number cross tabulation of the comparison between clinical grouping (CNP, MSP, pain free) and cluster grouping. As can be seen, the majority of the patients (72.9%) who were classified into Cluster 1= ‘hyposensitivity’ belonged to the CNP group, and the rest were divided between the MSP and pain‐free group. The majority of the patients (45.7%) who were classified into Cluster 2= ‘poor inhibition & hyperalgesia’ belonged to the MSP group, and the rest were equally divided between the CNP and pain‐free group. The majority of the patients (57.5%) who were classified into Cluster 3= ‘efficient inhibition’ belonged to the pain‐free MS group. This classification was significant at the 0.05 level.
TABLE 4.
Quantified presentation of the association of cluster membership and clinical groups
| Two step cluster number | Clinical groups | ||
|---|---|---|---|
|
CNP count (%) |
MSP count (%) |
pain free count (%) |
|
| Cluster 1 (n=37) | 27 (72.9)a | 3 (8.1)b | 7 (18.4)b |
| Cluster 2 (n=35) | 9 (25.7)a | 16 (45.7)b | 10 (28.6)a |
| Cluster 3 (n=40) | 8 (20.0)a | 9 (22.5)a | 23 (57.5)b |
CNP=MS patients with central neuropathic pain, MSP=MS patients with musculoskeletal pain, pain free=MS patients without pain. Two‐step number cross tabulation: each subscript letter (a,b) denotes a subset of clinical group categories whose column proportions (i.e., within clusters) do not differ significantly from each other at the 0.05 level (in bold are the unique groups within every cluster).
4. DISCUSSION
The results show that although the clinical characteristics of the two chronic pain groups were mostly similar, cluster analysis based on QST revealed their unique features.
4.1. Clinical features of CNP versus MSP
The CNP and the MSP groups were almost indistinguishable in chronic pain‐related features; average and highest chronic pain severity, pain duration and pain interference levels, as also reported for neuropathic and non‐neuropathic pain groups in neuromyelitis optica (NMO) (Valerio et al., 2020). Burning pain quality was more frequent among CNP than MSP patients, aligning with Svendsen et al. (2005). However, sharp pain and radiating pain were mutual qualities. The lower limbs were the most painful body regions for both CNP and MSP groups, aligning with Svendsen et al. and with others who studied MS patients with MSP (Kahraman et al., 2019; ShayestehAzar et al., 2015). Yet patients with CNP exhibited a more widespread chronic pain than those with MSP, and greater prevalence of headaches, which corresponds with Moisset et al. (2013). Both the chronic pain groups had a similar mechanical or cold allodynia frequency, consistent with Valerio et al. (2020), although those with CNP tended to have a somewhat greater allodynia rate, as reported by Svendsen et al. Importantly, although patients with CNP met its diagnostic criteria (Widerström‐Noga et al., 2017), the overlap in the pain characteristics between CNP and MSP challenges the distinction between them. This situation is particularly true for MS patients due to the diffuse spread of MS lesions, and hence of related symptoms.
The CNP and MSP groups also had similar MS‐related characteristics including EDSS, MS type and MS‐medication intake, corroborating previous reports (Boneschi, 2008; Kahraman et al., 2019; Kalia & O`Connor, 2005). The MSP group, however, had a longer disease duration similar to Kahraman et al. report. Given that MSP is often secondary to the MS manifestations, MSP may require a longer disease duration to emerge. Fewer patients in both the CNP and the MSP groups were employed compared with the pain‐free group, perhaps due to the added disability imposed by chronic pain (Grant et al., 2019). Both the CNP and the MSP groups had higher EDSS scores compared with the pain‐free group, in line with previous reports (Heitmann et al., 2015; Kahraman et al., 2019; Pompa et al., 2015; Truini et al., 2012) which may result from greater fatigue, disability and depression due to the pain (Heitmann, 2020; Kahraman et al., 2019). Nevertheless, EDSS or disease duration did not affect the differences between the groups in the sensory testing.
4.2. Sensory profile of CNP versus MSP
QST revealed significant differences between the CNP and MSP groups; innocuous thermal sensations and TGI thresholds were higher in the former, and the MSP group resembled the pain‐free group. Of note is that sensory thresholds among both groups were measured in the shins, which were also the most painful region and the region most sensory‐affected in both groups, suggesting these regions were comparable. Aligning with Srotova et al. (2020), we recently reported spinothalamic alterations among CNP patients compared to pain‐free MS patients (Rivel et al., 2021), which correspond with altered laser‐evoked potentials in these patients (Truini et al., 2012). Here we show that this quality occurs beyond the existence of chronic pain and of MS, and is unique to CNP. Of note is that only in the CNP group there was an asymmetry in sensory thresholds between painful and pain‐free body regions, as also reported by Österberg and Boivie (2010), which is an additional unique feature of this group. Svendsen et al. (2005) reported no differences in thermal and touch thresholds between CNP and MSP groups. However, our study defined CNP based on the diagnostic criteria of the American Pain Society Pain Taxonomy (AAPT), which did not exist in 2005, possibly explaining the inconsistency.
To our knowledge, the current study is the first, in which pain inhibition tests were compared between two chronic pain groups with MS. The CNP and MSP groups had similar pain adaptation and offset analgesia. Although the CNP group had worse pain adaptation than did the pain‐free group, offset analgesia was similar for these two groups, corresponding with Srotova et al. (2020) who reported conditioned pain modulation that was similar in CNP and pain‐free MS patients. Combined, the results suggest that although CNP in MS may be associated with deficient pain modulation, this trait may not be unique to CNP, but rather typifies chronic pain in general.
4.3. Distinct clusters among the MS cohort
Given the variability in QST among MS patients and the clinical overlap between the CNP and MSP groups, and considering the importance of QST in phenotyping pain conditions and studying their mechanisms (Arendt‐Nielsen & Yarnitsky, 2009; Backonja et al., 2013), QST‐based cluster analysis was applied. Three distinct clusters were identified: Cluster 1 was characterized by the highest thermal, TGI and mechanical thresholds, hence termed ‘hyposensitivity’ and reflected the CNP group. Cluster 2 was characterized by the poorest pain adaptation and offset analgesia, and by the lowest TGI threshold, hence termed ‘poor inhibition and hyperalgesia’. The majority of the patients in this cluster (although not the vast majority) belonged to the MSP group. Thus, although both CNP and MSP groups had alterations in thermal sensibility it was of an opposite direction: loss of function in the CNP group (Cluster 1), and some loss in the innocuous range but gain in the noxious range in the MSP group (Cluster 2). The classification of these patients into Clusters 1 or 2 may provide an additional approach in understanding the mechanisms of each of these chronic pain conditions, and may help in their differentiation.
Functional deficits in spinothalamic conduction were reported among MS patients with CNP based on a comparison with pain‐free patients (Rivel et al., 2021; Srotova et al., 2020) and are supported by the present findings in Cluster 1. Mechanical hypoalgesia, however, was previously reported for MS patients with chronic pain, but not specifically CNP (Fernández‐de‐Las‐Peñas et al., 2015). A sensory profile similar to that of Cluster 1 was reported for people with CNP due to other etiologies although not in all cases (e.gDefrin et al., 2001; Klit et al., 2011; Ofek & Defrin, 2007; Tuveson et al., 2009) and therefore may characterize a CNP subtype. MS lesions along the spinothalamic‐thalamocortical pathways, as well as in brain regions that receive their input, may lead to pathological processes in the vicinity of, and within, deafferented nociceptive neurons, and in turn, to CNP emergence (He et al., 2021; Poncet‐Megemont et al., 2019; Wu et al., 2013). Unexpectedly, however, poor pain inhibition did not characterize the CNP group in the cluster analysis, despite reports of poor inhibition among other CNP patients (Albu et al., 2015; Gruener et al., 2016, 2020; Naugle et al., 2020; Tuveson et al., 2009). However, these studies did not compare CNP patients with other chronic pain types and, therefore, whether their findings were unique to CNP was unclear.
Poor inhibition and thermal hyperalgesia (Cluster 2) characterized the majority of the MSP patients. A recent systematic review has concluded that poor CPM and hyperalgesia characterize multiple musculoskeletal conditions (Georgopoulos et al., 2019). These features can be associated with each other, but causative relations cannot be deduced from the present study. PA and OA reflect the function of endogenous pain inhibition mechanisms (D’Agata et al., 2015; Shulman et al., 2020; Zhang et al., 2018), the alterations of which – owing to MS‐lesions – may contribute to enhanced activity and reactivity of spinal nociceptive neurons, hence to centrally mediated hyperalgesia (Campbell & Meyer, 2006). Hyperalgesia can also result from sensitized nociceptors (Treed et al., 1992; Woolf, 2011), which in MS may occur due to secondary consequences of the disease, including poor posture and/or balance, muscle weakness and muscle spasm (Brola et al., 2014; Truini et al., 2013). At the same time, continuous hyperalgesia may also weaken inhibitory control. Either of these processes may lead to chronification of MSP in MS.
Pain‐free MS patients (Cluster 3) were characterized by some degree of thermal hypoesthesia along with very efficient pain inhibition, which was better than that found in healthy participants. Previous studies have reported that pain‐free MS patients exhibit some sensory alterations, although to a far lesser extent than those with CNP (Österberg & Boivie, 2010; Rivel et al., 2021; Srotova et al., 2020). Thus, while the damages in the somatosensory system of pain‐free MS patients may be insufficient to potentially induce neuropathic pain, their descending pain inhibition is highly efficient, a dual trait that may protect them from chronic pain (Arendt‐Nielsen et al., 2018; Georgopoulos et al., 2019). Of note, whereas the pain‐free group was younger than the two chronic pain groups, the mean age of the participants in Cluster 3 was higher than that of Cluster 1. Probably, the inclusion of patients with MSP increased the mean age of Cluster 3; however, the age range of the participants in the current study was not expected to induce any effects on the QST data (e.g., Lautenbacher et al., 2017).
Notably, the top three QST indices that best classified the three clusters were cold and warm sensation threshold, and offset analgesia. Thus, instead of conducting a full QST battery, perhaps these three indices are sufficient to distinguish between CNP and MSP in MS. This differentiation is particularly difficult among MS patients because of the nature of the disease: MS lesions can cause widespread damages in the somatosensory and motor systems, which in turn may lead to a plethora of symptoms that may resemble both CNP and MSP. Thus, in addition to the existing guidelines and diagnostic criteria for CNP or MSP in MS, an economical use of QST can help clinicians tailor individually based pain management.
4.4. Limitations and summary
Several limitations should be considered, first, although predetermining cluster number allowed for analysing the association with the three clinical groups, future studies, with larger samples, may favour automatic determination of cluster number. Second, although the clinical diagnosis of CNP and MSP was based on accepted criteria, the very nature of MS may affect the certainty of their diagnosis. Third, although the participants were cognitively capable of completing the sensory tests, response latencies and MS‐related cognitive alterations may have affected the QST data. Fourth, data were obtained from the most painful body region within each patient; however, this does not preclude the possibility that additional regions not covered by the testing were painful. In summary, whereas MS patients with CNP are characterized by functional interruption of the spinothalamic/thalamocortical pathways, those with MSP are characterized by hyperalgesia and poor pain inhibition; each unique profile can be a target for pain management interventions. Minor somatosensory alterations and very efficient pain inhibition may protect MS patients from chronic pain.
CONFLICTS OF INTEREST
There are no conflicts of interest.
Supporting information
Table S1
ACKNOWLEDGEMENTS
This study was conducted in partial fulfillment of the requirements for a PhD degree in Physical Therapy by Michal Rivel at the Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel. This work was partially supported by a grant from the National MS Society and by a grant from the Israeli Pain Association.
Rivel, M. , Achiron, A. , Dolev, M. , Stern, Y. , Zeilig, G. , & Defrin, R. (2022). Unique features of central neuropathic pain in multiple sclerosis: Results of a cluster analysis. European Journal of Pain, 26, 1107–1122. 10.1002/ejp.1934
REFERENCES
- Albu, S. , Gómez‐Soriano, J. , Avila‐Martin, G. , & Taylor, J. (2015). Deficient conditioned pain modulation after spinal cord injury correlates with clinical spontaneous pain measures. Pain, 156(2), 260–272. 10.1097/01.j.pain.0000460306.48701.f9 [DOI] [PubMed] [Google Scholar]
- Arendt‐Nielsen, L. , Morlion, B. , Perrot, S. , Dahan, A. , Dickenson, A. , Kress, H. G. , Wells, C. , Bouhassira, D. , & Mohr, D. A. (2018). Assessment and manifestation of central sensitisation across different chronic pain conditions. European Journal of Pain, 22(2), 216–241. 10.1002/ejp.1140 [DOI] [PubMed] [Google Scholar]
- Arendt‐Nielsen, L. , & Yarnitsky, D. (2009). Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. The Journal of Pain, 10(6), 556–572. 10.1016/j.jpain.2009.02.002 [DOI] [PubMed] [Google Scholar]
- Backonja, M. M. , Attal, N. , Baron, R. , Bouhassira, D. , Drangholt, M. , Dyck, P. J. , Edwards, R. R. , Freeman, R. , Gracely, R. , Haanpaa, M. H. , Hansson, P. , Hatem, S. M. , Krumova, E. K. , Jensen, T. S. , Maier, C. , Mick, G. , Rice, A. S. , Rolke, R. , Treede, R. D. , … Ziegler, D. (2013). Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain, 154(9), 1807–1819. 10.1016/j.pain.2013.05.047 [DOI] [PubMed] [Google Scholar]
- Baron, R. , Maier, C. , Attal, N. , Binder, A. , Bouhassira, D. , Cruccu, G. , Finnerup, N. B. , Haanpää, M. , Hansson, P. , Hüllemann, P. , Jensen, T. S. , Freynhagen, R. , Kennedy, J. D. , Magerl, W. , Mainka, T. , Reimer, M. , Rice, A. S. C. , Segerdahl, M. , Serra, J. , … Treede, R.‐D. (2017). Peripheral neuropathic pain: A mechanism‐related organizing principle based on sensory profiles. Pain, 158(2), 261–272. 10.1097/j.pain.0000000000000753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauch, E. M. , Andreou, C. , Rausch, V. H. , & Bunzeck, N. (2017). Neural habituation to painful stimuli is modulated by dopamine: Evidence from a pharmacological fMRI study. Frontiers in Human Neuroscience, 11, 630. 10.3389/fnhum.2017.00630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingel, U. , Schoell, E. , Herken, W. , Büchel, C. , Teutsch, S. , & May, A. (2007). Habituation to painful stimulation involves the antinociceptive system. Pain, 131, 21–30. 10.1016/j.pain.2006.12.005 [DOI] [PubMed] [Google Scholar]
- Boneschi, F. M. , Colombo, B. , Annovazzi, P. , Martinelli, V. , Bernasconi, L. , Solaro, C. , & Comi, G. (2008). Lifetime and actual prevalence of pain and headache in multiple sclerosis. Multiple Sclerosis, 14, 514–521. 10.1177/1352458507085551 [DOI] [PubMed] [Google Scholar]
- Bouhassira, D. , Attal, N. , Alchaar, H. , Boureau, F. , Brochet, B. , Brexelle, J. , Cunin, G. , Fermanian, J. , Ginies, P. , Grun‐OVerdyking, A. , Jafari‐Schluep, H. , Lantéri‐Minet, M. , Laurent, B. , Mick, G. , Serrie, A. , Valade, D. , & Vicaut, E. (2005). Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnosis questionnaire (DN4©). Pain, 114(1–2), 29–36. [DOI] [PubMed] [Google Scholar]
- Brola, W. , Mitosek‐Szewczyk, K. , & Opara, J. (2014). Symptomatology and pathogenesis of different types of pain in multiple sclerosis. Neurologia I Neurochirurgia Polska, 48(4), 272–279. 10.1016/j.pjnns.2014.07.009 [DOI] [PubMed] [Google Scholar]
- Campbell, J. N. , & Meyer, R. (2006). Mechanisms of neuropathic pain. Neuron, 52, 77–92. 10.1016/j.neuron.2006.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, A. D. , & Bushnell, M. C. (1994). The thermal grill illusion: Unmasking the burn of cold pain. Science, 265, 252–255. 10.1126/science.8023144 [DOI] [PubMed] [Google Scholar]
- D’Agata, F. , Cicerale, A. , Mingolla, A. , Caroppo, P. , Orsi, L. , Mortara, P. , Troni, W. , & Pinessi, L. (2015). Double‐cone coil TMS stimulation of the medial cortex inhibits central pain habituation. PLoS One, 10(6), e0128765. 10.1371/journal.pone.0128765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defrin, R. , Benstein‐Sheraizin, A. , Bezalel, A. , Mantzur, O. , & Arendt‐Nielsen, L. (2008). The spatial characteristics of the painful thermal grill illusion. Pain, 138, 577–586. 10.1016/j.pain.2008.02.012 [DOI] [PubMed] [Google Scholar]
- Defrin, R. , Ohry, A. , Blumen, N. , & Urca, G. (2001). Characterization of chronic pain and somatosensory function in spinal cord injury subjects. Pain, 89, 253–263. 10.1016/S0304-3959(00)00369-9 [DOI] [PubMed] [Google Scholar]
- Derbyshire, S. W. G. , & Osborn, J. (2008). Enhancement of offset analgesia during sequential testing. European Journal of Pain, 12, 980–989. 10.1016/j.ejpain.2008.01.008 [DOI] [PubMed] [Google Scholar]
- Dodt, E. , & Zotterman, Y. (1952). Mode of action of warm receptors. Acta Physiologica Scandinavica, 26(4), 345–357. 10.1111/j.1748-1716.1952.tb00916.x [DOI] [PubMed] [Google Scholar]
- Doth, A. H. , Hansson, P. T. , Jensen, M. P. , & Taylor, R. S. (2010). The burden of neuropathic pain: A systematic review and meta‐analysis of health utilities. Pain, 149, 338–344. 10.1016/j.pain.2010.02.034 [DOI] [PubMed] [Google Scholar]
- Failde, I. , Dueñas, M. , Ribera, M. V. , Gálvez, R. , Mico, J. A. , Salazar, A. , de Sola, H. , & Pérez, C. (2018). Prevalence of central and peripheral neuropathic pain in patients attending pain clinics in Spain: Factors related to intensity of pain and quality of life. Journal of Pain Research, 11, 1835–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feketová, S. , Waczulíková, I. , Valkovič, P. , & Mareš, J. (2017). Central pain in patients with multiple sclerosis. Journal of Multiple Sclerosis, 4, 208. [Google Scholar]
- Fernández‐de‐Las‐Peñas, C. , Ortega‐Santiago, R. , Ortíz‐Gutiérrez, R. , Caminero, A. B. , Salom‐Moreno, J. , & Arendt‐Nielsen, L. (2015). Widespread pressure pain hypersensitivity in patients with multiple sclerosis with and without pain as sign of central sensitization. The Clinical Journal of Pain, 31(1), 66–72. 10.1097/AJP.0000000000000084 [DOI] [PubMed] [Google Scholar]
- Ferraro, D. , Plantone, D. , Morselli, F. , Dallari, G. , Simone, A. M. , Vitetta, F. , Sola, P. , Primiano, G. , Nociti, V. , Pardini, M. , Mirabella, M. , & Vollono, C. (2018). Systematic assessment and characterization of chronic pain in multiple sclerosis patients. Neurological Sciences, 39, 445–453. 10.1007/s10072-017-3217-x [DOI] [PubMed] [Google Scholar]
- Foley, P. L. , Vesterinen, H. M. , Laird, B. J. , Sena, E. S. , Colvin, L. A. , Chandran, S. , MacLeod, M. R. , & Fallon, M. T. (2013). Prevalence and natural history of pain in adults with multiple sclerosis: Systematic review and meta‐analysis. Pain, 154(5), 632–642. 10.1016/j.pain.2012.12.002 [DOI] [PubMed] [Google Scholar]
- Gelbard, R. , Goldman, O. , & Spiegler, I. (2007). Investigating diversity of clustering methods: An empirical comparison. Data and Knowledge Engineering, 63, 155–166. 10.1016/j.datak.2007.01.002 [DOI] [Google Scholar]
- Georgopoulos, V. , Akin‐Akinyosoye, K. , Zhang, W. , McWilliams, D. F. , Hendrick, P. , & Walsh, D. A. (2019). Quantitative sensory testing and predicting outcomes for musculoskeletal pain, disability, and negative affect: A systematic review and meta‐analysis. Pain, 160(9), 1920–1932. 10.1097/j.pain.0000000000001590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, M. , Rees, S. , Underwood, M. , & Froud, R. (2019). Obstacles to returning to work with chronic pain: In‐depth interviews with people who are off work due to chronic pain and employers. BMC Musculoskeletal Disorders, 20(1), 486. 10.1186/s12891-019-2877-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso, M. G. , Clemenzi, A. , Tonini, A. , Pace, L. , Casillo, P. , Cuccaro, A. , Pompa, A. , & Troisi, E. (2008). Pain in multiple sclerosis: a clinical and instrumental approach. Multiple Sclerosis, 14, 506–513. 10.1177/1352458507085553 [DOI] [PubMed] [Google Scholar]
- Grill, J. D. , & Coghil, R. C. (2002). Transient analgesia evoked by noxious stimulus offset. Journal of Neurophysiology, 87, 2205–2208. 10.1152/jn.00730.2001 [DOI] [PubMed] [Google Scholar]
- Gruener, H. , Zeilig, G. , Gaidukov, E. , Rachamim‐Katz, O. , Ringler, E. , Blumen, N. , Engel‐Haber, E. , & Defrin, R. (2020). Biomarkers for predicting central neuropathic pain occurrence and severity after spinal cord injury: Results of a long‐term longitudinal study. Pain, 161(3), 545–556. 10.1097/j.pain.0000000000001740 [DOI] [PubMed] [Google Scholar]
- Gruener, H. , Zeilig, G. , Laufer, Y. , Blumen, N. , & Defrin, R. (2016). Differential pain modulation properties in central neuropathic pain after spinal cord injury. Pain, 157, 1415–1424. 10.1097/j.pain.0000000000000532 [DOI] [PubMed] [Google Scholar]
- He, C. , Liu, R. , Fan, Z. , Li, Y. , Yang, M. , Wugang, H. , Lu, Z. , Fang, Z. , & Su, B. (2021). Microglia in the pathophysiology of hemorrhagic stroke and the relationship between microglia and pain after stroke: A narrative review. Pain and Therapy. 10(2), 927–939. 10.1007/s40122-021-00288-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitmann, H. , Biberacher, V. , Tiemann, L. , Buck, D. , Loleit, V. , Selter, R. C. , Knier, B. , Tölle, T. R. , Mühlau, M. , Berthele, A. , Hemmer, B. , & Ploner, M. (2015). Prevalence of neuropathic pain in early multiple sclerosis. Multiple Sclerosis Journal, 22(9), 1224–1230. 10.1177/1352458515613643 [DOI] [PubMed] [Google Scholar]
- Heitmann, H. , Haller, B. , Tiemann, L. , Mühlau, M. , Berthele, A. , Tölle, T. R. , Salmen, A. , Ambrosius, B. , Bayas, A. , Asseyer, S. , Hartung, H. P. , Heesen, C. , Stangel, M. , Wildemann, B. , Haars, S. , Groppa, S. , Luessi, F. , Kümpfel, T. , Nischwitz, S. , … Ploner, M. (2020). German competence network multiple sclerosis (KKNMS). Longitudinal prevalence and determinants of pain in multiple sclerosis: Results from the German National Multiple Sclerosis Cohort study. Pain, 161(4):787–796. [DOI] [PubMed] [Google Scholar]
- Iggo, A. (1969). Cutaneous thermoreceptors in primates and sub‐primates. The Journal of Physiology, 200, 403–430. 10.1113/jphysiol.1969.sp008701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutzeler, C. R. , Warner, F. M. , Wanek, J. , Curt, A. , & Kramer, J. L. K. (2017). Thermal grill conditioning: Effect on contact heat evoked potentials. Scientific Reports, 7, 1–8. 10.1038/srep40007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahraman, T. , Özdoğar, A. T. , Ertekin, Ö. , & Özakbaş, S. (2019). Frequency, type, distribution of pain and related factors in persons with multiple sclerosis. Multiple Sclerosis and Related Disorders, 28, 221–225. 10.1016/j.msard.2019.01.002 [DOI] [PubMed] [Google Scholar]
- Kalia, L. V. , & OConnor, P. W. (2005). Severity of chronic pain and its relationship to quality of life in multiple sclerosis. Multiple Sclerosis, 11, 322–327. 10.1191/1352458505ms1168oa [DOI] [PubMed] [Google Scholar]
- Kaufman, L. , & Rousseeuw, P. J. (2005). Finding groups in data: An introduction to cluster analysis. Wiley. [Google Scholar]
- Klit, K. , Finnerup, N. B. , Andersen, G. , & Jensen, T. S. (2011). Central poststroke pain: A population‐based study. Pain, 152, 818–824. 10.1016/j.pain.2010.12.030 [DOI] [PubMed] [Google Scholar]
- Lautenbacher, S. , Peters, J. H. , Heesen, M. , Scheel, J. , & Kunz, M. (2017). Age changes in pain perception: A systematic‐review and meta‐analysis of age effects on pain and tolerance thresholds. Neuroscience and Biobehavioral Reviews, 75, 104–113. 10.1016/j.neubiorev.2017.01.039 [DOI] [PubMed] [Google Scholar]
- Melzack, R. (1975). The McGill Pain Questionnaire: Major properties and scoring methods. Pain, 1(3), 277–299. 10.1016/0304-3959(75)90044-5 [DOI] [PubMed] [Google Scholar]
- Moisset, X. , Ouchchane, L. , Guy, N. , Bayle, D. J. , Dallel, R. , & Clavelou, P. (2013). Migraine headaches and pain with neuropathic characteristics: Comorbid conditions in patients with multiple sclerosis. Pain, 154(12), 2691–2699. [DOI] [PubMed] [Google Scholar]
- Naugle, K. M. , Carey, C. , Evans, E. , Saxe, J. , Overman, R. , & White, F. A. (2020). The role of deficient pain modulatory systems in the development of persistent post‐traumatic headaches following mild traumatic brain injury: An exploratory longitudinal study. The Journal of Headache and Pain, 21(1), 138. 10.1186/s10194-020-01207-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noback, C. R. , Strominger, N. L. , Demarest, R. J. , & Ruggiero, D. A. (2012). In Strominger N. L., Demarest R. J., Laemle L. B. (Eds.), Noback’s, 7th Edn. Human nervous system, structure and function (pp. 177–192). Springer Science+Business Media. [Google Scholar]
- O’Connor, A. B. , Schwid, S. R. , Herrmann, D. N. , Markman, J. D. , & Dworkin, R. H. (2008). Pain associated with multiple sclerosis: Systematic review and proposed classification. Pain, 137, 96–111. 10.1016/j.pain.2007.08.024 [DOI] [PubMed] [Google Scholar]
- Ofek, H. , & Defrin, R. (2007). The characteristics of chronic central pain after traumatic brain injury. Pain, 131(3), 330–340. 10.1016/j.pain.2007.06.015 [DOI] [PubMed] [Google Scholar]
- Österberg, A. , & Boivie, J. (2010). Central pain in multiple sclerosis‐sensory abnormalities. European Journal of Pain, 14, 104–110. 10.1016/j.ejpain.2009.03.003 [DOI] [PubMed] [Google Scholar]
- Österberg, A. , Boivie, J. , & Thuomas, K. A. (2005). Central pain in multiple sclerosis‐ prevalence and clinical characteristics. European Journal of Pain, 9, 531–542. 10.1016/j.ejpain.2004.11.005 [DOI] [PubMed] [Google Scholar]
- Polman, C. H. , Reingold, S. C. , Banwell, B. , Clanet, M. , Cohen, J. A. , Filippi, M. , Fujihara, K. , Havrdova, E. , Hutchinson, M. , Kappos, L. , Lublin, F. D. , Montalban, X. , O’Connor, P. , Sandberg‐Wollheim, M. , Thompson, A. J. , Waubant, E. , Weinshenker, B. , & Wolinsky, J. S. (2010). revisions to the McDonald criteria. American Neurological Association, 2011(69), 292–302. 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompa, A. , Clemenzi, A. , Troisi, E. , Pace, L. , Casillo, P. , Catani, S. , & Grasso, M. G. (2015). Chronic pain in multiple sclerosis patients: utility of sensory quantitative testing in patients with fibromyalgia comorbidity. European Neurology, 73, 257–263. [DOI] [PubMed] [Google Scholar]
- Poncet‐Megemont, L. , Dallel, R. , Chassain, C. , Perrey, A. , Mathais, S. , Clavelou, P. , & Moisset, X. (2019). Whole‐body reversible neuropathic pain associated with right parieto‐temporal operculum single inflammatory lesion in a patient with multiple sclerosis: A case report. European Journal of Pain, 23(10), 1763–1766. 10.1002/ejp.1464 [DOI] [PubMed] [Google Scholar]
- Rivel, M. , Achiron, A. , Dolev, M. , Stern, Y. , Zeilig, G. , & Defrin, R. (2021). Central neuropathic pain in multiple sclerosis is associated with impaired innocuous thermal pathways and neuronal hyperexcitability. Pain Medicine, 22(10), 1–13. 10.1093/pm/pnab103 [DOI] [PubMed] [Google Scholar]
- Scherder, R. J. , Kant, N. , Wolf, E. T. , Pijnenburg, B. C. M. , & Scherder, E. J. A. (2018). Sensory function and chronic pain in multiple sclerosis. Pain Research and Management, 1924174, 1–9. 10.1155/2018/1924174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ShayestehAzar, M. , Kariminasab, M. H. , Saravi, M. S. , Abedini, M. , Fazli, M. , Abbas, S. , Hashemi, S. A. , & Abdizadeh, P. (2015). A survey of severity and distribution of musculoskeletal pain in multiple sclerosis patients; a cross‐sectional study. Archives of Bone and Joint Surgery, 3(2), 114–118. [PMC free article] [PubMed] [Google Scholar]
- Shulman, J. , Zurakowski, D. , Keysor, J. , Jervis, K. , & Sethna, N. F. (2020). Offset analgesia identifies impaired endogenous pain modulation in pediatric chronic pain disorders. Pain, 161(12), 2852–2859. 10.1097/j.pain.0000000000001984 [DOI] [PubMed] [Google Scholar]
- Srotova, I. , Kocica, J. , Vollert, J. , Kolcava, J. , Hulova, M. , Jarkovsky, J. , Dusek, L. , Bednarik, J. , & Vlckova, E. (2020). Sensory and pain modulation profiles of ongoing central neuropathic extremity pain in multiple sclerosis. European Journal of Pain, 00, 1–22. [DOI] [PubMed] [Google Scholar]
- Svendsen, K. B. , Jensen, T. S. , Hansen, H. J. , & Bach, F. W. (2005). Sensory function and quality of life in patients with multiple sclerosis and pain. Pain, 114(4), 473–481. 10.1016/j.pain.2005.01.015 [DOI] [PubMed] [Google Scholar]
- Treede, R. D. , Meyer, R. A. , Raja, S. N. , & Campbell, J. N. (1992). Peripheral and central mechanisms of cutaneous hyperalgesia. Progress in Neurobiology, 38, 397–421. 10.1016/0301-0082(92)90027-C [DOI] [PubMed] [Google Scholar]
- Truini, A. , Barbanti, P. , Pozzilli, C. , & Cruccu, G. (2013). A mechanism‐based classification of pain in multiple sclerosis. Journal of Neurology, 260, 351–367. 10.1007/s00415-012-6579-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truini, A. , Galeotti, F. , La Cesa, S. , Di Rezze, S. , Biasiotta, A. , Di Stefano, G. , Tinelli, E. , Millefiorini, E. , Gatti, A. , & Cruccu, G. (2012). Mechanisms of pain in multiple sclerosis: A combined clinical and neurophysiological study. Pain, 153, 2048–2054. 10.1016/j.pain.2012.05.024 [DOI] [PubMed] [Google Scholar]
- Tuveson, B. , Leffler, A. S. , & Hansson, P. (2009). Influence of heterotopic noxious conditioning stimulation on spontaneous pain and dynamic mechanical allodynia in central post‐stroke pain patients. Pain, 143(1–2), 84–91. 10.1016/j.pain.2009.02.002 [DOI] [PubMed] [Google Scholar]
- Valerio, F. , Apostolos‐Pereira, S. L. , Sato, D. K. , Callegaro, D. , Lucato, L. T. , Barboza, V. R. , Silva, V. A. , Galhardoni, R. , de Lima Rodrigues, A. L. , Teixeira, M. J. , & de Andrade, D. C. (2020). Characterization of pain syndromes in patients with neuromyelitis optica. European Journal of Pain, 24(8), 1548–1568. 10.1002/ejp.1608 [DOI] [PubMed] [Google Scholar]
- Widerström‐Noga, E. , Loeser, J. D. , Jensen, T. S. , & Finnerup, N. B. (2017). AAPT diagnostic criteria for central neuropathic pain. The Journal of Pain, 18(20), 1417–1426. 10.1016/j.jpain.2017.06.003 [DOI] [PubMed] [Google Scholar]
- Willis, W. D. , & Westlund, K. N. (1997). Neuroanatomy of the pain system and of the pathways that modulate pain. Journal of Clinical Neurophysiology, 14(1), 2–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf, C. J. (2011). Central sensitization: Implications for the diagnosis and treatment of pain. Pain, 152(3), S2–S15. 10.1016/j.pain.2010.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J. , Raver, C. , Piao, C. , Keller, A. , & Faden, A. I. (2013). Cell cycle activation contributes to increased neuronal activity in the posterior thalamic nucleus and associated chronic hyperesthesia after rat spinal cord contusion. Neurotherapeutics: the Journal of the American Society for Experimental NeuroTherapeutics, 10(3), 520–538. 10.1007/s13311-013-0198-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilig, G. , Enosh, S. , Rubin‐Asher, D. , Lehr, B. , & Defrin, R. (2012). The nature and course of sensory changes following spinal cord injury: Predictive properties and implications on the mechanism of central pain. Brain, 135, 418–430. 10.1093/brain/awr270 [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Li, T. , Kobinata, H. , Ikeda, E. , Ota, T. , & Kurata, J. (2018). Attenuation of offset analgesia is associated with suppression of descending pain modulatory and reward systems in patients with chronic pain. Molecular Pain, 14, 1–15. 10.1177/1744806918767512 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


