Figure 1.
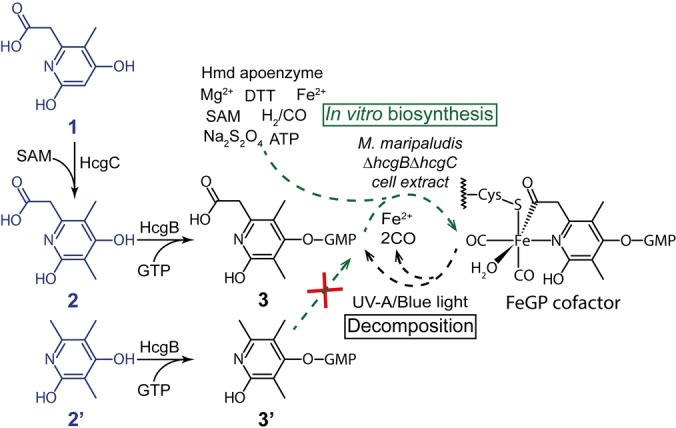
A part of the proposed biosynthetic sequence (from 1 to 3) and in vitro biosynthesis of the FeGP cofactor. The pyridinol derivatives (1, 2 and 2′ in blue) were chemically synthesized. The enzyme reactions, which convert the pyridinol compounds, are shown. 2′ and 3′ did not function as a precursor of in vitro biosynthesis. The acyl group of the FeGP cofactor is hydrolysed to the carboxy group of 3 by light decomposition.
