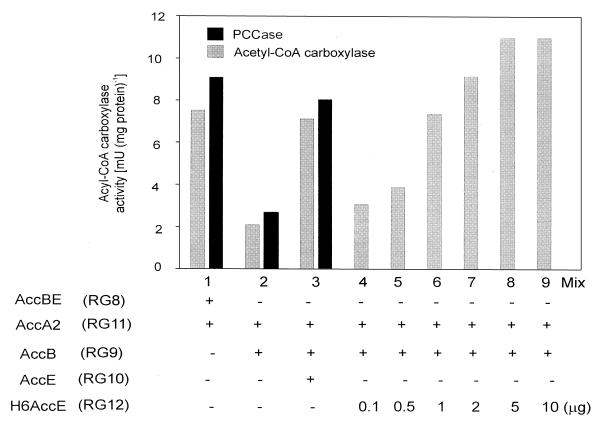
FIG. 2.
Effect of AccE on the catalytic activity of the ACCase complex. In mixes 1, 2, and 3, acetyl-CoA carboxylase and PCCase activities were measured after mixing equal amounts of proteins from cell extracts from each of the strains indicated. In mixes 4 to 9, ACCase activity was determined using a mix of RG9 and RG11 cell extracts containing different amounts of purified H6AccE. Results are the means of three determinations. When ACCase activity was measured in individual cell extracts, the amount of 14C fixed into acid-stable products was not significantly higher than background levels (10 cpm, equivalent to 0.02 mU).