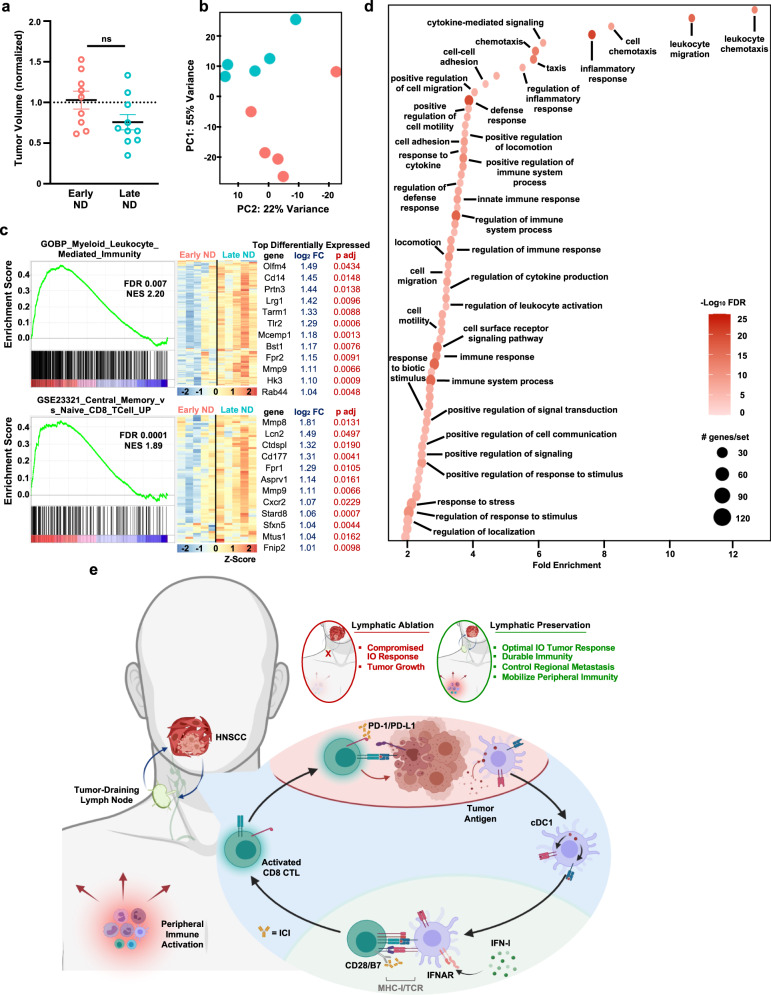
Fig. 6. Lymphatic-sparing IO therapy mobilizes peripheral antitumor immunity.
a Normalized tumor volumes on day 9 or 10 after orthotopic transplantation of 106 4MOSC1 tumor cells and treatment in vivo with two doses of αCTLA-4 followed by either an early (red, n = 9) or late (blue, n = 10) neck dissection—day 6 versus day 11. b Principal component analysis plot from whole blood RNA sequencing, performed at day 10 after transplantation of 106 4MOSC1 tumor cells and treatment in vivo with two doses of αCTLA-4 followed by either an early (red) or late (blue) neck dissection—day 6 versus day 11, calculated and plotted with DESeq2, n = 5. c Left: Representative gene set enrichment mountain plots of differentially expressed genes; and right: corresponding heatmaps depicting the row normalized Z-score of the top differentially expressed genes from those gene sets identified in analysis of whole blood RNA sequencing after transplantation of 106 4MOSC1 tumor cells and treatment in vivo with two doses of αCTLA-4, followed by either an early (red), or late (blue) neck dissection. d Bubble plot illustrating the top hits from a Gene Ontology analysis, illustrating GO hits enriched in late vs early ND treatment sequencing groups as described in Fig. 5a (log2FC > 1, adjusted P value < 0.05 upregulated genes identified in analysis of whole blood RNA sequencing; n = 5). e Cartoon describing the central role that the tumor-draining lymph node plays in the response to ICI therapy and the outcome of rational, lymphatic-preserving treatment sequencing in HNSCC; see text for details. The differences between experimental groups were analyzed using independent, two-sided t tests (a), DESeq2 (c) or Log2FC P < 0.05 (d). All data represent averages ± SEM, except where indicated. ns not statistically significant. Source data are provided as a Source Data file.