Abstract
Background
Continuous glucose monitoring (CGM) decreases fear of hypoglycemia (FOH) and improves glycemic control among those affected by type 1 diabetes (T1D). No studies to date have examined the impact of using do‐it‐yourself real‐time continuous glucose monitoring (DIY RT‐CGM) on psychological and glycemic outcomes.
Methods
Child–parent dyads were recruited for a multicentre randomized crossover trial. Children with T1D were current intermittently scanned CGM (isCGM) users and aged 2–13 years. Families received either 6 weeks of DIY RT‐CGM with parental remote monitoring (intervention) or 6 weeks of isCGM plus usual diabetes care (control), followed by a 4‐week washout period, then crossed over. The primary outcome was parental FOH. Secondary outcomes were glycemic control using traditional CGM metrics, as well as a range of other psychosocial measures.
Findings
Fifty five child–parent dyads were recruited. The child mean age was 9.1 ± 2.8 years. Although, there was no effect on parental FOH, −0.1 (95%CI: −0.3, 0.1, p = 0.4), time‐in‐range (TIR) (%3.9‐10 mmol/L) was significantly higher with DIY RT‐CGM over isCGM (54.3% ± 13.7 vs. 48.1% ± 13.6), mean difference, 5.7% (95%CI 1.8, 9.6, p <0.004). There was no difference for time spent in hypoglycemia. Parent diabetes treatment satisfaction was significantly higher following DIY RT‐CGM compared to isCGM, mean difference 5.3 (95%CI: 2.3, 8.2, p <0.001).
Conclusion
The use of DIY RT‐CGM versus isCGM did not improve parental FOH; however, TIR and parental satisfaction with diabetes treatment were significantly improved. This suggests in the short term, DIY RT‐CGM appears safe and may offer families some clinically important advantages over isCGM.
Keywords: continuous glucose monitoring, do‐it‐yourself, fear of hypoglycemia, type 1 diabetes
1. INTRODUCTION
Type 1 diabetes (T1D) is one of the most common chronic diseases of childhood. 1 Management of T1D is multifaceted and aims at controlling the risk of both acute and chronic complications as well as alleviating the impact of the disease and its management on the quality of life of affected children and their caregivers. 2 In addition, inadequate control of glucose levels and glucose variability, can have detrimental effect on brain development in children; thus improving time in range may help improve outcomes in this age group. 3 , 4 , 5 Current evidence‐based guidelines recommend intensive management with insulin of T1D to reduce and prevent diabetes‐related complications. 2 However, despite this focus on intensive management, many children with T1D still have control above current glycemic targets. 6 An additional price of intensive management can be an increased incidence of hypoglycaemia. 1 This increase in hypoglycemia is compounded by increased or additive fear of hypoglycemia (FOH), such FOH is influenced by severity, frequency, and timing of hypoglycemia, as well as longer duration of diabetes, and suboptimal diabetes control. 7 , 8 FOH is also associated with suboptimal glycaemic control for people affected by T1D, particularly children and their families across their life span. 7 , 8 , 9
Real‐time continuous glucose monitoring (RT‐CGM) has the ability to provide real‐time glucose data as well as parental remote monitoring of children with T1D. 10 , 11 In addition it may lead to improvements in glycemic control as well as some relief from FOH in patients and their caregivers. 10 , 12 , 13 On the other hand, RT‐CGM is expensive, and is not funded in many countries. 10 , 14 Intermittently scanned CGM (isCGM) is a cheaper alternative technology which provides many of the “on‐demand” benefits of RT‐CGM. 11 However, standard first generation isCGM does not have safety alerts/glucose threshold alarms, nor offers continuous remote monitoring. 11 Subsequent generations of isCGM (Abbott FreeStyle Libre 2 and 3) may address some of these issues but are currently not widely available and no evidence is available on their effectiveness. Overall, current data supports RT‐CGM being superior and more effective than isCGM in improving impaired awareness of hypoglycemia and in increasing time spent in the target glucose range. 15 , 16 However, studies comparing both technologies (RT‐CGM and isCGM) in pediatric population are lacking.
A third‐party device (MiaoMiao) (MiaoMiao version 2, Smart Reader, Shanghai High Brilliant Health Technology Co. Ltd., China), is also available. 17 The device is a Bluetooth transmitter and is designed to be placed over the standard isCGM sensor. 17 Using near‐field communication (NFC), MiaoMiao reads raw data from the isCGM sensor before sending it via Bluetooth™ to a paired smart device (usually the user's phone). 17 Driven by lower cost compared to other commercial RT‐CGM devices (MiaoMiao is available online for $US170 as a one‐off upfront cost), some patients are adopting this new technology as an affordable do‐it‐yourself (DIY) RT‐CGM alternative. 18 However, evidence on the effectiveness and the safety of DIY RT‐CGM is limited to anecdotal report 18 with no trial evidence to date. With the increasing number of users worldwide, research is clearly needed. To our knowledge, this is the first study to investigate the effectiveness of an isCGM system converted to DIY RT‐CGM in comparison to standard isCGM alone in children with T1D.
2. RESEARCH DESIGN AND METHODS
This randomized controlled study was conducted through district health boards (DHBs) across New Zealand (Southern, Capital and Coast, and Auckland DHBs) from November 2019 to October 2020. The study protocol was approved by the Northern Health and Disability Ethics Committee (19/NTB/118; Wellington, New Zealand). The study was registered with the Australian New Zealand Clinical Trials Registry (ACTRN 12619001551189) and was issued a Universal Trial Number (U1111‐1236‐9189) by the World Health Organization International Clinical Trials Registry. Neither the isCGM nor the MiaoMiao manufacturers were involved in the planning, funding, or conduct of the study. Children and one of their parents were recruited after being invited via their usual pediatric endocrinologist/ diabetes specialist during routine clinical visits. Children were eligible to take part in this study if: they were between 2 and 13 years old; had T1D for at least 6 months; were already using isCGM with the intention to continue using it for the whole study period; and were planning to continue with routine clinical care during the study period. Children were excluded from the study if they were already using DIY RT‐CGM or another RT‐CGM device; had any severe diabetes related complications; had severe uncontrolled medical or psychiatric co‐morbidities; and if they were in another study that could affect glucose measurements.
Full protocol details of this multisite 17‐week crossover study have been previously published. 19 In brief, the study recruited 55 child–parent dyads and involved a run‐in period for 1 week to collect basic participant and diabetes demographics. The sample size estimation used data available from previous studies (SD = 20 for parent‐reported hypoglycaemia fear survey [HFS], and a within‐person correlation of 0.52 20 , 21 ) to determine that 50 participants were needed to have 80% power to detect a difference of 8 points on HFS between the two treatments with a two‐sided α of 0.05. In order to allow for 10% dropout it was aimed to recruit 55 families. Eligible families were randomly allocated to one of two groups: to use DIY RT‐CGM with remote monitoring (intervention) or to continue using standard isCGM (FreeStyle Libre [Abbott Diabetes Care Ltd., Witney, UK]) (control) for a 6‐week phase. This was followed by a washout phase of 4 weeks before all participants crossed over to the other arm for the second 6 week phase. Randomization for intervention order was stratified by study site and was computer generated using the Sealed Envelope website (http://www.sealedenvelope.com/).
Participants in the DIY RT‐CGM phase of the study were given a comprehensive education package provided by a member of the research team (MME) while participants in the control phase were followed up for any problem with isCGM sensor or reader. The MiaoMiao device allowed the transmission of glucose values from the FreeStyle Libre sensor via Bluetooth to an application on the child's phone. The information was shared via the “cloud” to the same application on their parents' phones, who were then able to monitor their child's CGM readings in real‐time remotely. For the purpose of this study the xDrip+ application 22 was downloaded on both child and parent phones during the education visit (after the run‐in period or after the wash‐out period). xDrip+ works as a data processor and hub that supports connection between different smart phones, sensors as well as smart watches. A smartwatch (Fitbit Versa Lite, Fitbit Inc., San Francisco, California, USA) was also used for children over 8 years to receive glucose measurements. Alerts were offered for each child's phone and their smart watch (if worn). We recommended low and high alerts of 3.9 and 15 mmol/L (70 and 270 mg/dl) respectively. However, families, in conjunction with the research team, were permitted to make threshold changes according to their preferences at study commencement and during the study period. Due to restrictions imposed during the Covid‐19 pandemic, most of the study visits were done online using secured Zoom video meetings (Zoom Video Communications, Inc., San Jose, California, USA).
Once/day calibrations of the system were also recommended to support system accuracy preferably in the morning before breakfast when glucose levels were stable. As an additional safety precaution, caregivers and children were recommended to perform finger prick glucose confirmations prior to therapeutic interventions. The calibrated glucose reading was entered in xDrip+. Both the intervention and the control groups otherwise received their prestudy standard diabetes care and the same clinical follow up. Their usual clinical team, rather than the research team, were responsible for all the diabetes management advice, other than device technical support; therefore, the study did not provide additional advise on insulin dosing.
2.1. Outcome measures
At four time points: baseline, after the wash‐out period, and at completion of the first and the second 6‐week treatment periods, parents and children (aged 6–13 years) were asked to complete validated questionnaires, and glycemic measures were collected. Parental FOH was assessed by using HFS, which includes two subscales: Behavior (10 items) and Worry (15 items). 23 Children were also asked, with support of one of the research team as needed, to complete the HFS with the same subscale structure, and this constituted a secondary outcome for the study. Quality of life was assessed by the Pediatric Quality of Life Inventory (PedsQL) modules: the PedsQL Generic Core (version 4.0), and the PedsQL Inventory Diabetes Module (version 3.2). 24 Both previous modules included a parent, a proxy‐report for ages 2–13, and children self‐report for ages 5–13 (version 4.0). Parent quality of life was investigated using the PedsQL Family Impact Module (version 2.0). 25 Parental satisfaction with their child's treatment was assessed by the diabetes treatment satisfaction questionnaire (DTSQ), using both status and change versions. 26 User acceptability was assessed by an in‐house developed non‐standardized user experience questionnaire, which was consisted of a five point Likert scale: 1 (strongly disagree), 2 (Disagree), 3 (neutral), 4 (agree), and 5 (strongly agree). All questionnaires were collected from one parent who attended all the study visits and who was primarily involved in his/her child's diabetes care (self‐nominated). All participants were asked to upload their isCGM readings at baseline to Tidepool. 27 Both participants and researchers used Tidepool as the standard platform for isCGM readings for the control group and to collect continuous glucose data for the DIY RT‐CGM group. The study glycemic variables included; time in target range 3.9–10 mmol/L (70–180 mg/dl), time spent >10 mmol/L (>180 mg/dl), time spent >13.9 mmol/L (>250 mg/dl), time spent in hypoglycemia <3.9 mmol/L (<70 mg/dl), and time spent in hypoglycemia <3.0 mmol/L (<54 mg/dl), all recorded as percent of the total time (6 weeks). Mean glucose as well as % coefficient of variation (%CV) were also calculated.
3. ROLE OF THE FUNDING SOURCE
The funding sources had no role in study design, data collection, data analysis, interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
4. STATISTICAL ANALYSIS
The analysis of the primary and secondary outcomes was completed on an intention‐to‐treat principle with patients included in the analysis of the group they were allocated into. To assess the difference between outcomes after the intervention phase compared to the control phase, mixed linear regression models were used with a random effect for participant and adjusted for the “baseline” measure taken at the start of the study. Residuals of all models were plotted and visually assessed for heteroskedasticity and normality. If skewed outcome data were influencing model assumptions, these data were log‐transformed and mean differences reported as percent difference. Analyses were adjusted to randomization order to test for carry‐over effect. Results were not adjusted for multiple comparisons. Statistical analysis was blinded to the randomization and group allocation of participants. Stata 16.1 (StataCorp, Texas, USA) was used for statistical analysis. A two‐sided p value < 0.05 was considered statistically significant.
5. RESULTS
In total, 55 child–parent dyads were recruited. Participant flow through the study is provided in Figure 1. Complete data were available for 50 families (91%). Patient baseline demographic characteristics are reported in Table 1. The median age of participating children was 9.5 years, ranging from 3 to 13 years, while the mean age of parents was 40.5 years (range 28–53). The mean duration of T1D was 36.2 months (range 6.5–115).
FIGURE 1.
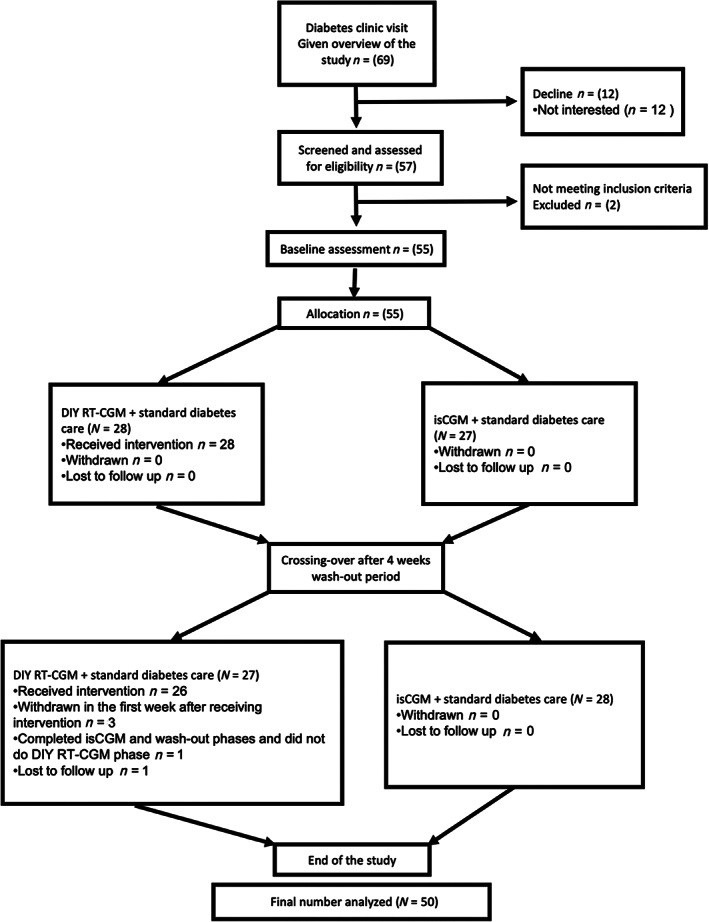
Consolidated standard of reporting trial (CONSORT) participant flow diagram
TABLE 1.
Baseline demographics for parents and their children (n = 55)
| Parents | |
|---|---|
| Age (years), mean ± SD | 40.5 ± 6.1 |
| Sex, n (%) | |
| Female | 51.0 (92.7) |
| Ethnicity, n (%) | |
| European | 45.0 (81.8) |
| Māori a | 2.0 (3.6) |
| Pacific Islander | 2.0 (3.6) |
| Other b | 6.0 (10.9) |
| NZDep13 c , n (%) | |
| Low deprivation (1–3) | 25.0 (45.4) |
| Medium deprivation (4–7) | 21.0 (38.2) |
| High deprivation (8–10) | 9.0 (16.4) |
| Education, n (%) | |
| Some high school | 8.0 (14.5) |
| High school | 10.0 (18.2) |
| Further training after high school | 4.0 (7.3) |
| Tertiary | 25.0 (45.4) |
| Postgraduate qualifications d | 8.0 (14.5) |
| Employment, n (%) | |
| Full time | 22.0 (40.0) |
| Part time | 28.0 (50.9) |
| Full time carer | 5.0 (9.1) |
| Marital status, n (%) | |
| Married | 40.0 (72.7) |
| Separated | 3.0 (5.4) |
| Partner/civil union | 10.0 (18.2) |
| Single | 2.0 (3.6) |
| Children | |
|---|---|
| Age (years), mean ± SD | 9.1 ± 2.8 |
| Sex, n (%) | |
| Female | 31.0 (56.3) |
| Male | 24.0 (43.6) |
| BMI z‐score, mean ± SD | 0.7 ± 1.0 |
| Duration of diabetes (months), mean ± SD | 36.2 ± 25.6 |
| Insulin therapy, n (%) | |
| MDI | 34.0 (61.8) |
| CSII | 21.0 (38.2) |
| Insulin estimated total daily dose (units), mean ± SD | 29.4 ± 17.8 |
| Duration of using isCGM (months), mean ± SD | 18.2 ± 13.7 |
| HbA1c %, mean ± SD | 7.6 ± 3.2 |
| HbA1c mmol/mol, mean ± SD | 59.8 ± 11.1 |
Abbreviations: CSII, continuous subcutaneous insulin infusion; MDI, multiple daily injections.
Māori are the indigenous people of New Zealand.
Chinese (n = 1) and different ethnicity (n = 5).
NZDep13 score is a deprivation index based on household address (where the participant lives more than 50% of the time) with 1 least deprived and 10 most deprived.
Postgraduate qualifications are postgraduate degrees such as a postgraduate diploma, Master or a PhD.
5.1. Fear of hypoglycemia, quality of life, and diabetes treatment satisfaction
Results for HFS, quality of life and diabetes treatment satisfaction instruments are all fully presented in Table 2 and Supplementary Table 1. In short, no significant differences were seen between treatment arms in either parental or child HFS scores and sub‐scales. No significant differences were seen in parental PedsQL Generic total score. However, the quality of life subscale related to physical functioning showed a statistically significant improvement in favor of the DIY RT‐CGM (mean difference 6.1, 95% CI [2.0:10.2], p = 0.003) and the same pattern was seen in children (mean difference 6.0, 95% CI [1.0:10.9], p = 0.018).
TABLE 2.
Effect of DIY RT‐CGM on fear of hypoglycemia and glycemic outcomes compared to isCGM (n = 50)
| HFS c (parent) | isCGM a | DIY RT‐CGM b mean ± SD | Mean Difference (95% CI) | |||
|---|---|---|---|---|---|---|
| Baseline | Six weeks | Baseline | Six weeks | |||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | p value | ||
| Behavior subscale | 2.5 ± 0.6 | 2.3 ± 0.6 | 2.4 ± 0.6 | 2.2 ± 0.5 | −0.1 (−0.2:0.1) | 0.440 |
| Worry subscale | 1.7 ± 0.8 | 1.6 ± 0.7 | 1.7 ± 0.8 | 1.4 ± 0.6 | −0.1(−0.3:0.1) | 0.387 |
| Total score | 2.0 ± 0.6 | 1.9 ± 0.6 | 2.0 ± 0.6 | 1.7 ± 0.5 | −0.1 (−0.3:0.1) | 0.354 |
| HFS c (child) | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
|---|---|---|---|---|---|---|
| Behavior subscale | 2.1 ± 0.5 | 2.1 ± 0.5 | 2.1 ± 0.6 | 2.1 ± 0.5 | −0.0 (−0.2:0.2) | 0.729 |
| Worry subscale | 1.2 ± 0.7 | 1.1 ± 0.7 | 1.2 ± 0.7 | 1.2 ± 0.7 | 0.1 (−0.1:0.3) | 0.232 |
| Total score | 1.6 ± 0.5 | 1.5 ± 0.5 | 1.5 ± 0.6 | 1.5 ± 0.5 | 0.1 (−0.1:0.2) | 0.477 |
| Glycaemic outcomes | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean difference (95% CI) | p value |
|---|---|---|---|---|---|---|
| % time in target 3.9–10 mmol/L (70–180 mg/dl) | 47.2 ± 16.4 | 48.1 ± 13.6 | 46.7 ± 15.7 | 54.3 ± 13.7 | 5.7 (1.8:9.6) | 0.004 |
| % time spent <3.9 mmol/L (<70 mg/dl) d | 3.3 (1.9,7.0) | 4.0 (2.0, 7.0) | 4.0 (2.0, 7.0) | 3.0 (1.2, 4.0) | −20.0% (−38.0%:3.0%) | 0.083 |
| % time spent <3 mmol/L (<54 mg/dl) d | 1.0 (0.1, 2.5) | 1.0 (0, 2.0) | 1.0 (0.1, 3.0) | 0.4 (0.2, 1.0) | −14.0% (−30.0%: 6.0%) | 0.158 |
| % time spent >10 mmol/L (>180 mg/dl) | 47.7 ± 18.2 | 46.8 ± 15.7 | 48.4 ± 16.8 | 41.4 ± 15.1 | −4.6 (−9.3:‐0.0) | 0.047 |
| % time spent >13.9 mmol/L (>250 mg/dl) | 22.3 ± 15.6 | 21.4 ± 13.0 | 22.8 ± 14.8 | 15.7 ± 9.8 | −6.7 (−10.3:‐3.1) | 0.001 |
| Mean glucose, mmol/L | 10.2 ± 1.9 | 10.2 ± 1.6 | 10.2 ± 1.6 | 9.8 ± 1.1 | −0.49 (−0.93, −0.04) | 0.032 |
| %CV | 41.0 ± 6.9 | 42.6 ± 7.0 | 40.1 ± 6.0 | 38.8 ± 5.5 | −3.2 (−5.5, −0.9) | 0.007 |
Note: Glycemic data from 14‐day xDrip+/Tidepool extractions. Only one family used Tomato app but final data extraction was done through Tidepool.
isCGM intermittently scanned Continuous Glucose Monitoring.
DIY RT‐CGM Do‐It‐Yourself Real‐Time Continuous Glucose Monitoring.
Hypoglycemia Fear Survey (HFS); higher scores reflect higher levels of tendency to avoid or worry about hypoglycemia.
Data are presented as median (range). Differences are presented as percentage difference due to transformation of skewed data.
On average, the total score of family quality of life improved for both arms, but improved significantly more after the DIY RT‐CGM (mean difference: 3.8, 95% CI [0.8:6.8], p = 0.014). In addition, this improvement was found in the Parent Health Related Quality of Life (HRQL) subscale score.
Parental diabetes treatment satisfaction as measured by DTSQc, also improved for DIY RT‐CGM compared to isCGM (mean difference 5.2, 95% CI [2.3:8.2], p = 0.001).
5.2. Glycemic outcomes
Detailed data are provided in Table 2. An improvement in percentage time spent in target glucose range (TIR, 3.9–10 mmol/L [70–180 mg/dl]) was seen after using the DIY RT‐CGM. Baseline TIR increased from 46.7% ± 15.7% to 54.3% ± 13.7% at intervention end, compared with 46.2% ± 16.4% to 48.1% ± 13.6% with isCGM. The mean difference between both groups was 5.7% (95% CI 1.8:9.6), p = 0.004.
Corresponding decreases were seen in percentage of time spent in hyperglycemia (>10 mmol/L [>180 mg/dl]), and severe hyperglycemia (>13.9 mmol/L [>250 mg/dl]) after using DIY RT‐CGM (mean difference −4.6 95% CI (−9.3:−0.0), p value 0.047, and −6.7 95% CI (−10.3:‐3.1), p = 0.001, respectively). No significant differences were seen between DIY RT‐CGM and isCGM for time spent in hypoglycemia (<3.9 mmol/L), [<70 mg/dl], or severe hypoglycemia (<3 mmol/L), [<54 mg/dl].
An analysis of the proportion of participants who met TIR and TBR targets (≥70% and <4% of time, respectively) is shown in Supplementary Figure 2. The results show that very few participants were meeting TIR ≥70%, but MiaoMiao seemed to improve the likelihood of this (14% compared to 4%). It is also shown that 44% of the sample were meeting TBR <4% after isCGM but 64% were meeting this target after MiaoMiao, indicating that the intervention improved the likelihood of meeting hypo‐targets.
A subgroup analysis investigated the effectiveness of DIY RT‐CGM compared to isCGM by those using CDII and MDI. FOH in both parents and children did not differ markedly between those using CSII or MDI (parental fear: 0.0 (−0.3, 0.3) (n = 18) in CSII whereas with MDI it was −0.1 (−0.3, 0.1) (n = 32); child fear: 0.0 (−0.2, 0.2) (n = 16) whereas with MDI it was 0.1 (−0.1, 0.3) (n = 25). However, DIY RT‐CGM compared to isCGM was considerably more effective for increasing TIR in children using MDI compared to those using CSII (−1.1% (−6.8, 4.5) (n = 18) for CSII whereas with MDI it was 9.6% (4.8, 14.4) (n = 32).
5.3. Acceptance of DIY RT‐CGM
Using a non‐standardized user experience questionnaire, 94% of our participants found wearing DIY RT‐CGM was easy, and 67% trusted the readings from the device. As reported by their caregivers, most of the children (82%) did not mind having DIY RT‐CGM on top of their isCGM sensor. In addition, most children (86%) did not find that the device got in the way of their daily activities. Most caregivers (92%) found remote monitoring of their children's blood glucose levels helpful and 71% reported having more freedom due to remote monitoring. The majority of caregivers (82%) planned to continue using the DIY RT‐CGM at study completion and all 51 families who completed the study reported that they would recommend using DIY RT‐CGM for other families. Further data are provided in the supplementary Figures 1–3.
Loss of connection to DIY RT‐CGM was one of the main problems reported by participants with 76% reporting loss of connection between child's phone and the DIY RT‐CGM. 72% of parents reported loss of the follower function on their phone. The mean estimated self‐reported % loss‐of‐signal time was 17% for the master device and 21% for the follower device. During DIY RT‐CGM use, glucose threshold alerts were turned off at some stage by 39% of participants during the day and 31% during the night.
The incidence of adverse events (AE) and serious adverse events (SAE) were recorded by the investigators. No AE or SAE were recorded during the intervention phase with the use of the DIY RT‐CGM. There were no reported severe skin reactions, episodes of diabetic ketoacidosis nor severe hypoglycaemia, or hospital admission due to complications of T1D during the study. In addition, it should be noted that no episodes of severe hypoglycaemia were experienced by any participant in the 12 months prior to the study.
6. DISCUSSION
This randomized controlled crossover study provides much needed effectiveness data for DIY RT‐CGM use in children. The primary outcome, parental fear of hypoglycaemia, showed no evidence of a difference between isCGM and DIY RT‐CGM. However, some psychological parameters of the pediatric quality of life family impact module, pediatric generic physical functioning subscale for both parents and their children and the DTSQ did improve. Importantly, glycemic control as measured by traditional metrics of TIR and time spent in hyperglycemia did show both clinical and statistically significant improvements with DIY RT‐CGM over isCGM. Overall, time in hypoglycemia was similar with both interventions, and while DIY RT‐CGM did show small reductions in TBR, these were not statistically significant, this is possibly reflected in the FOH negative outcome. This improvement in TIR and time spent in hyperglycemia may be of particular importance in this younger age group due to the potentially damaging impacts of hyperglycemia on neuro‐developmental aspects. 3 , 4 These data are critical as families worldwide have adopted this cheaper RT‐CGM technology with no evidence of effectiveness to date. 18
To our knowledge, this is the first study designed and powered to investigate the impact of using novel DIY RT‐CGM devices in children with T1D and their parents/caregivers. We report both clinical and statistically significant improvements in glycemic control as measured by TIR. This is the first study to present glycemic data for DIY RT‐CGM and contributes to the overall literature comparing RT‐CGM to isCGM especially with no randomized controlled studies available in children. A recent randomized study conducted in adults with T1D revealed that the use of RT‐CGM for 4 weeks improved TIR as compared to isCGM, with the authors noting benefits from alarms/glucose alerts. 16 Additionally, the I Hart CGM study presented data from two studies comparing isCGM to RT‐CGM in adults at risk of severe hypoglycaemia. 15 , 28 Their extension phase data also suggested benefits to TIR, as well as benefits to hypoglycaemia frequency 28 in their high‐risk population, which we did not find in children. Interestingly, our overall improvements in TIR are also comparable to recent trials in an older age groups for commercial non‐adjunctive RT‐CGM compared to capillary testing. 29 , 30
FOH is an important management aspect in patients with T1D and their caregivers, adds to disease burden and can be a barrier to achieving healthy clinical outcomes. 6 , 8 , 31 Previous research using traditional RT‐CGM has highlighted improvements in parental and child FOH. 12 , 32 Our findings of no difference may be explained by differences between commercial RT‐CGM and the DIY system and can be coupled with the fact that FOH was not high at baseline for these included children and their caregivers with fairly well‐controlled T1D. Rates of hypoglycemia were also comparable and minimal for both interventions throughout the study. DIY technology, as highlighted in both the literature 18 and in our data, is prone to connectivity and integration issues, these occurred for many in this study despite considerable technical support as needed from the research team. Interestingly, glucose threshold alerts were also frequently silenced by participants, and while it is uncertain exactly why this occurred, clearly this reflects the real world experience of this technology, and possibly alarm fatigue as previously described 33 . However, our findings are consistent with those from the Diabetes Research in Children Network (DirectNet), which did not report a significant improvement in parental FOH after using RT‐CGM in a similar cohort of young children. 20 In addition, the Juvenile Diabetes Research foundation (JDRF) study found no evidence of a difference in parental FOH with the use of RT‐CGM in comparison to the use of standard blood glucose monitoring in 451 children and adults with T1D. 34 Interestingly, children between 8 and 17 years and their parents reported greater anxiety after the use of RT‐CGM in a small follow‐up study to the JDRF trial. 35 However, the results of both the DirectNet and the JDRF studies might be explained by the limited technology of the used sensor and the lack of remote monitoring availability at that time. A recent study showed no difference in FOH between CGM and capillary blood glucose management in very young children with T1D; and only RT‐CGM combined with a family behavioral intervention could achieve improvements in FOH. 36 We did not assess the remote monitoring component of the DIY‐RT CGM separately, but it might have a role in the improvement in the quality of life of caregivers as shown in the Nightscout users' survey. 37
Again, while the impact of using commercial RT‐CGM on psychosocial parameters has previously been investigated in comparison to finger prick capillary glucose, little data is available for RT‐CGM versus isCGM, and none available for DIY systems. We highlight that, similar to commercial RT‐CGM, parental quality of life, family functioning, and diabetes treatment satisfaction improved with the DIY system even when compared to isCGM, an arguably superior comparator to intermittent capillary glucose. 12 In addition, we report that both the physical functioning for parents and children measured as a part of PedsQL generic questionnaires showed a significant improvement, suggesting that DIY RT‐CGM may be superior to isCGM for these aspects. Otherwise, our findings agree with the JDRF study, which reported no meaningful change in generic or diabetes‐related quality of life. 34 We also report a high degree of parental satisfaction after the use of DIY RT‐CGM compared to isCGM. This was despite relatively frequent technical and connectivity issues reported. All of the above may provide additional evidence on potential superiority of RT‐CGM to isCGM in these contexts.
Strengths of this study include the robust randomized crossover design. High retention was also a strength and suggests the DIY system was largely well tolerated. However, it was not always easy or practical for this cohort of young children to manage carrying the multiple devices required for DIY RT‐CGM (phone, smart watch, glucometer, and in some case insulin pump), which may be a limitation of the DIY system itself. This is supported by the fact that device burden and connectivity issues were the reasons given for why three families with children <6 years decided to leave the study within the first week of the DIY intervention. These issues are likely to carry less burden with commercial vs. DIY systems. There are limitations regarding FOH for this study. These include the strong comparator (isCGM) with regards to preventing hypoglycemia, as well as overall low‐baseline FOH in those recruited, and low rates of hypoglycemia in both arms. The nonsignificant change in FOH in our study could also be impacted by the relatively short duration of the intervention. These limitation could be addressed in future studies in populations experiencing more FOH and overall at higher risk for hypoglycemia. Finally, while our data provide much‐needed initial evidence regarding DIY‐CGM safety, these systems are not registered and are unregulated. There is currently also an FDA warning regarding a reported AE with this system, and this study in no way addresses ongoing debates around issues such as patient free‐choice, and unresolved legal liability, and medical indemnity.
In conclusion, this is the first randomized controlled crossover trial in any population with T1D to investigate the effectiveness of DIY RT‐CGM on psychological and glycemic outcomes. We highlight improvements in glycemic outcomes, quality of life, and diabetes treatment satisfaction that are very similar to established commercial CGM technologies, and highlight some potential benefits of RT‐CGM over isCGM in this age group. Importantly, the current study highlights the viability, relative short‐term safety, and challenges of using DIY RT‐CGM in children with T1D, a technology being used worldwide, but to date with little supporting evidence.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Benjamin J Wheeler is the guarantor of this work. Mona M Elbalshy, Sara Styles, Barbara C Galland, Hamish Crocket, Craig Jefferies, Esko Wiltshire, Paul Tomlinson, Martin I de Bock, and Benjamin J Wheeler were involved in the design of the study protocol. Mona M Elbalshy provided the full technical support and education. Jillian J. Haszard did the statistical analysis for this study. Mona M Elbalshy, Sara Styles, Barbara C Galland, Hamish Crocket, Craig Jefferies, Esko Wiltshire, Paul Tomlinson, Martin I de Bock, and Benjamin J Wheeler were involved with the recruitment process and data collection. Mona M Elbalshy wrote the first draft of the manuscript. All authors participated in reviewing the final manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/pedi.13331.
Supporting information
Supplementary Figure 1 User experience regarding putting on do‐it‐yourself real‐time continuous glucose monitoring (DIY RT‐CGM). “Easy to put on” shows the users experience regarding applying DIY RT‐CGM to FreeStyle Libre sensor. “Adhesive effective” is showing the users experience regarding how effective the adhesive between FreeStyle Libre and DIY RT‐CGM
Supplementary Figure 2: Proportions of participants within TIR and TBR targets before and after treatments (n = 50). Blue bars represent being within range (TIR ≥ 70% or TBR < 4% of the time) after the treatment. Red bars represent being out of range after treatment. The light colors represent those who were within range at baseline. 4% meeting the TIR target of ≥70% of time after the isCGM treatment, compared to 14% after DIY RT‐CGM (blue bars). 44% meeting the hypo‐target of <4% of time after the isCGM treatment, compared to 64% after DIY RT‐CGM (blue bars). 30% of the sample were not meeting the hypo‐target before treatment but were meeting it after DIY RT‐CGM, compared to only 4% with isCGM (dark blue bars)
Supplementary Figure 3: Overall user experience of using do‐it‐yourself realtime continuous glucose monitoring (DIY RT‐CGM) in comparison to FreeStyle Libre. The figure shows how users would compare different aspects of using DIY RT‐CGM to FreeStyle Libre including DIY RT‐CGM being more accurate “More accurate,” DIY RT‐CGM is more user friendly “User friendly,” children would not mind having DIY RT‐CGM on “Child doesn't mind,” DIY RT‐CGM did not bother parents/caregivers “DIY RT‐CGM didn't bother me,” DIY RT‐CGM does not cause skin reactions “no skin reaction,” DIY RT‐CGM does not have a negative impact on child's activities “no effect on activities,” DIY RT‐CGM had lower impact on child's social life “Less socially intrusive,” DIY RT‐CGM readings on master phone was more helpful “DIY RT‐CGM more helpful” and DIY RT‐CGM remote monitoring was more helpful “Remote monitoring helpful,” DIY RT‐CGM alarms were helpful “Alarms helpful,” DIY RT‐CGM made T1D management easier “Treatment easier,” DIY RT‐CGM increased users interest in treatment of their children's T1D “Better interest in treatment,” DIY RT‐CGM improved relation with child “Better relation with child,” and DIY RT‐CGM gave parents/caregivers more freedom “Gave me more freedom”
Supplementary Table 1: Effect of DIY RT‐CGM on psychosocial outcomes compared to isCGM (n = 50).
ACKNOWLEDGMENTS
Open access publishing facilitated by University of Otago, as part of the Wiley ‐ University of Otago agreement via the Council of Australian University Librarians. Add a correction statement: [Correction added on 1 June 2022 after first online: CAUL funding statement has been added.]
Elbalshy MM, Styles S, Haszard JJ, et al. The effect of do‐it‐yourself real‐time continuous glucose monitoring on psychological and glycemic variables in children with type 1 diabetes: A randomized crossover trial. Pediatr Diabetes. 2022;23(4):480‐488. doi: 10.1111/pedi.13331
Funding information Department of Women's and Children's Health Research Grant; Dunedin School of Medicine Research Grant; Freemasons New Zealand; Health Research Council of New Zealand; Lottery Health Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. DiMeglio LA, Evans‐Molina C, Oram RA. Type 1 diabetes. The Lancet. 2018;391(10138):2449‐2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care. 2009;32(1):187‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Backestrom A, Papadopoulos K, Eriksson S, et al. Acute hyperglycaemia leads to altered frontal lobe brain activity and reduced working memory in type 2 diabetes. PLoS One. 2021;16(3):e0247753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mauras N, Buckingham B, White NH, et al. Impact of type 1 diabetes in the developing brain in children: a longitudinal study. Diabetes Care. 2021;44(4):983‐992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593‐1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abraham MB, Jones TW, Naranjo D, et al. ISPAD clinical practice consensus guidelines 2018: assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes. 2018;19:178‐192. [DOI] [PubMed] [Google Scholar]
- 7. Driscoll KA, Raymond J, Naranjo D, Patton SR. Fear of hypoglycemia in children and adolescents and their parents with type 1 diabetes. Curr Diab Rep. 2016;16(8):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haugstvedt A, Wentzel‐Larsen T, Graue M, Søvik O, Rokne B. Fear of hypoglycaemia in mothers and fathers of children with type 1 diabetes is associated with poor glycaemic control and parental emotional distress: a population‐based study. Diabet Med. 2010;27(1):72‐78. [DOI] [PubMed] [Google Scholar]
- 9. Adolfsson P, Rentoul D, Klinkenbijl B, Parkin CG. Hypoglycaemia remains the key obstacle to optimal Glycaemic control–continuous glucose monitoring is the solution. Eur Endocrinol. 2018;14(2):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Funtanilla VD, Caliendo T, Hilas O. Continuous glucose monitoring: a review of available systems. P T. 2019;44(9):550. [PMC free article] [PubMed] [Google Scholar]
- 11. Srivastava R, Kumar S, Jyoti B, Kumar R. FreeStyle® libre™ flash glucose monitoring system: a novel diagnostic technique for monitoring diabetes. Int J Contemporary Med Surg Radiol. 2018;3(3):C48‐C52. [Google Scholar]
- 12. Burckhardt M‐A, Roberts A, Smith GJ, Abraham MB, Davis EA, Jones TW. The use of continuous glucose monitoring with remote monitoring improves psychosocial measures in parents of children with type 1 diabetes: a randomized crossover trial. Diabetes Care. 2018;41(12):2641‐2643. [DOI] [PubMed] [Google Scholar]
- 13. DeSalvo DJ, Keith‐Hynes P, Peyser T, et al. Remote glucose monitoring in cAMP setting reduces the risk of prolonged nocturnal hypoglycemia. Diabetes Technol Ther. 2014;16(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 14. Kraaijeveld SR. Continuous glucose monitoring as a matter of justice. HEC Forum. 2021;33(4):345–370. doi: 10.1007/s10730-020-09413-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reddy M, Jugnee N, El Laboudi A, Spanudakis E, Anantharaja S, Oliver N. A randomized controlled pilot study of continuous glucose monitoring and flash glucose monitoring in people with type 1 diabetes and impaired awareness of hypoglycaemia. Diabet Med. 2018;35(4):483‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hásková A, Radovnická L, Petruželková L, et al. Real‐time CGM is superior to flash glucose monitoring for glucose control in type 1 diabetes: the CORRIDA randomized controlled trial. Diabetes Care. 2020;43(11):2744‐2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. MIAOMIAO , Smart Reader for freestyle libre, continuous glucose readings every 5 minutes straight to your mobile phone or watch, 2020. https://miaomiao.cool/ 2020.
- 18. Elbalshy M, Boucher S, Crocket H, et al. Exploring parental experiences of using a do‐it‐yourself solution for continuous glucose monitoring among children and adolescents with type 1 diabetes: a qualitative study. J Diabetes Metab Disord. 2020;14(5):844‐853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elbalshy M, Boucher S, Galland B, et al. The MiaoMiao study: can do‐it‐yourself continuous glucose monitoring technology improve fear of hypoglycaemia in parents of children affected by type 1 diabetes? J Diabetes Metab Disord. 2020;20:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mauras N, Beck R, Xing D, et al. A randomized clinical trial to assess the efficacy and safety of real‐time continuous glucose monitoring in the management of type 1 diabetes in young children aged 4 to< 10 years. Diabetes Care. 2012;35(2):204‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Green LB, Wysocki T, Reineck BM. Fear of hypoglycemia in children and adolescents with diabetes. J Pediatr Psychol. 1990;15(5):633‐641. [DOI] [PubMed] [Google Scholar]
- 22. NightscoutFoundation/xDrip "Nightscout xDrip+,” xDrip & Nightscout: https://github.com/NightscoutFoundation/xDrip. 2021.
- 23. Gonder‐Frederick L, Nyer M, Shepard JA, Vajda K, Clarke W. Assessing fear of hypoglycemia in children with type 1 diabetes and their parents. Diabetes Manag. 2011;1(6):627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Varni JW, Burwinkle TM, Jacobs JR, Gottschalk M, Kaufman F, Jones KL. The PedsQL™ in type 1 and type 2 diabetes: reliability and validity of the pediatric quality of life inventory™ generic core scales and type 1 diabetes module. Diabetes Care. 2003;26(3):631‐637. [DOI] [PubMed] [Google Scholar]
- 25. Varni JW, Sherman SA, Burwinkle TM, Dickinson PE, Dixon P. The PedsQL™ family impact module: preliminary reliability and validity. Health Qual Life Outcomes. 2004;2(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bradley C. Diabetes treatment satisfaction questionnaire. Diabetes Care. 1999;22(3):530. [DOI] [PubMed] [Google Scholar]
- 27. About Tidepool . 2020. https://www.tidepool.org/about.
- 28. Reddy M, Jugnee N, Anantharaja S, Oliver N. Switching from flash glucose monitoring to continuous glucose monitoring on hypoglycemia in adults with type 1 diabetes at high hypoglycemia risk: the extension phase of the I HART CGM study. Diabetes Technol Ther. 2018;20(11):751‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thabit H, Prabhu JN, Mubita W, et al. Use of factory‐calibrated real‐time continuous glucose monitoring improves time in target and HbA1c in a multiethnic cohort of adolescents and young adults with type 1 diabetes: the MILLENNIALS study. Diabetes Care. 2020;43(10):2537‐2543. [DOI] [PubMed] [Google Scholar]
- 30. Laffel LM, Kanapka LG, Beck RW, et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323(23):2388‐2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalra S, Mukherjee JJ, Venkataraman S, et al. Hypoglycemia: the neglected complication. Indian J Endocrinol Metab. 2013;17(5):819‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ng SM, Moore HS, Clemente MF, Pintus D, Soni A. Continuous glucose monitoring in children with type 1 diabetes improves well‐being, alleviates worry and fear of hypoglycemia. Diabetes Technol Ther. 2019;21(3):133‐137. [DOI] [PubMed] [Google Scholar]
- 33. Farfel A, Liberman A, Yackobovitch‐Gavan M, Phillip M, Nimri R. Executive functions and adherence to continuous glucose monitoring in children and adolescents with type 1 diabetes. Diabetes Technol Ther. 2020;22(4):265‐270. [DOI] [PubMed] [Google Scholar]
- 34. Lawrence JM, Laffel L, Wysocki T, et al. Quality of life measures in children and adults with type 1 diabetes: the juvenile diabetes research foundation continuous glucose monitoring randomized trial. Diabetes Care. 2010;33(10):2175‐2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Markowitz JT, Pratt K, Aggarwal J, Volkening LK, Laffel LMB. Psychosocial correlates of continuous glucose monitoring use in youth and adults with type 1 diabetes and parents of youth. Diabetes Technol Ther. 2012;14(6):523‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Strategies to Enhance New CGMUiECSG . A randomized clinical trial assessing continuous glucose monitoring (CGM) use with standardized education with or without a family behavioral intervention compared with fingerstick blood glucose monitoring in very young children with type 1 diabetes. Diabetes Care. 2021;44(2):464‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee JM, Newman MW, Gebremariam A, et al. Real‐world use and self‐reported health outcomes of a patient‐designed do‐it‐yourself mobile technology system for diabetes: lessons for mobile health. Diabetes Technol Ther. 2017;19(4):209‐219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 User experience regarding putting on do‐it‐yourself real‐time continuous glucose monitoring (DIY RT‐CGM). “Easy to put on” shows the users experience regarding applying DIY RT‐CGM to FreeStyle Libre sensor. “Adhesive effective” is showing the users experience regarding how effective the adhesive between FreeStyle Libre and DIY RT‐CGM
Supplementary Figure 2: Proportions of participants within TIR and TBR targets before and after treatments (n = 50). Blue bars represent being within range (TIR ≥ 70% or TBR < 4% of the time) after the treatment. Red bars represent being out of range after treatment. The light colors represent those who were within range at baseline. 4% meeting the TIR target of ≥70% of time after the isCGM treatment, compared to 14% after DIY RT‐CGM (blue bars). 44% meeting the hypo‐target of <4% of time after the isCGM treatment, compared to 64% after DIY RT‐CGM (blue bars). 30% of the sample were not meeting the hypo‐target before treatment but were meeting it after DIY RT‐CGM, compared to only 4% with isCGM (dark blue bars)
Supplementary Figure 3: Overall user experience of using do‐it‐yourself realtime continuous glucose monitoring (DIY RT‐CGM) in comparison to FreeStyle Libre. The figure shows how users would compare different aspects of using DIY RT‐CGM to FreeStyle Libre including DIY RT‐CGM being more accurate “More accurate,” DIY RT‐CGM is more user friendly “User friendly,” children would not mind having DIY RT‐CGM on “Child doesn't mind,” DIY RT‐CGM did not bother parents/caregivers “DIY RT‐CGM didn't bother me,” DIY RT‐CGM does not cause skin reactions “no skin reaction,” DIY RT‐CGM does not have a negative impact on child's activities “no effect on activities,” DIY RT‐CGM had lower impact on child's social life “Less socially intrusive,” DIY RT‐CGM readings on master phone was more helpful “DIY RT‐CGM more helpful” and DIY RT‐CGM remote monitoring was more helpful “Remote monitoring helpful,” DIY RT‐CGM alarms were helpful “Alarms helpful,” DIY RT‐CGM made T1D management easier “Treatment easier,” DIY RT‐CGM increased users interest in treatment of their children's T1D “Better interest in treatment,” DIY RT‐CGM improved relation with child “Better relation with child,” and DIY RT‐CGM gave parents/caregivers more freedom “Gave me more freedom”
Supplementary Table 1: Effect of DIY RT‐CGM on psychosocial outcomes compared to isCGM (n = 50).
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


