Abstract
Objective
To estimate the timing of cannabidiol (CBD) treatment effect (seizure reduction and adverse events [AEs]) onset, we conducted a post hoc analysis of GWPCARE6 (NCT02544763), a randomized, placebo‐controlled, phase 3 trial in patients with drug‐resistant epilepsy associated with tuberous sclerosis complex (TSC).
Methods
Patients received plant‐derived pharmaceutical formulation of highly purified CBD (Epidiolex; 100 mg/ml oral solution) at 25 mg/kg/day (CBD25) or 50 mg/kg/day (CBD50) or placebo for 16 weeks (4‐week titration, 12‐week maintenance). Treatment started at 5 mg/kg/day for all groups and reached 25 mg/kg/day on Day 9 and 50 mg/kg/day on Day 29. Percentage change from baseline in TSC‐associated seizure (countable focal or generalized) count was calculated by cumulative day (i.e., including all previous days). Time to onset and resolution of AEs were evaluated.
Results
Of 224 patients, 75 were randomized to CBD25, 73 to CBD50, and 76 to placebo. Median (range) age was 11.3 (1.1–56.8) years. Patients had discontinued a median (range) of 4 (0–15) antiseizure medications and were currently taking 3 (0–5). Difference in seizure reduction between CBD and placebo emerged on Day 6 (titrated dose, 15 mg/kg/day) and became nominally significant (p < .049) by Day 10. Separation between placebo and CBD in ≥50% responder rate also emerged by Day 10. Onset of AEs occurred during the first 2 weeks of the titration period in 61% of patients (CBD25, 61%; CBD50, 67%; placebo, 54%). In patients with an AE, resolution occurred within 4 weeks of onset in 42% of placebo and 27% of CBD patients and by end of trial in 78% of placebo and 51% of CBD patients.
Significance
Onset of treatment effect occurred within 6–10 days. AEs lasted longer for CBD than placebo, but the most common (diarrhea, decreased appetite, and somnolence) resolved during the 16‐week trial in most patients.
Keywords: antiseizure medication, cannabidiol, epilepsy, focal seizures, medication‐resistant seizures, tuberous sclerosis complex
Key Points.
This post hoc analysis assessed the timing of CBD treatment effect onset in patients with TSC‐associated epilepsy from the GWPCARE6 trial
Patients received placebo or CBD, titrated up to 25 mg/kg/day or 50 mg/kg/day; all patients reached the 25‐mg dose on Day 9
Greater reduction in seizures with CBD versus placebo emerged by Day 6 (nominal significance by Day 10) and was maintained through the trial
Onset of AEs occurred within 2 weeks of starting treatment in 64% of patients on CBD and 54% on placebo
AEs resolved by trial end in most patients with diarrhea (88%), decreased appetite (83%), and somnolence (69%)—the most frequent AEs
1. INTRODUCTION
Tuberous sclerosis complex (TSC) is a genetic disorder caused primarily by mutations in tumor suppressor genes TSC1 or TSC2, resulting in increased activation of the mechanistic target of rapamycin (mTOR) pathway, with subsequent excessive cell growth and proliferation. 1 , 2 , 3 , 4 TSC is characterized by benign hamartomas in multiple organ systems, especially brain, skin, kidneys, lungs, heart, and eyes. 1 , 2 , 3 , 5 Epilepsy is a common neurologic manifestation, occurring in approximately 85% of patients with TSC. 6 , 7 , 8 , 9 , 10 Onset of seizures usually occurs during the first 2 years of life and can persist lifelong, with multiple seizure types. 6 , 7 , 8 , 9 , 10 Patients often have infantile spasms and focal seizures as infants; however, as the disease progresses, they may experience other seizure types. 8 , 11 Early onset and severe epilepsy are associated with higher rates of autism spectrum disorder and intellectual disability. 12
Seizures associated with TSC are currently treated with a range of antiseizure medications (ASMs), the mTOR pathway inhibitor everolimus, surgical procedures, and dietary therapy. 6 , 13 However, despite these treatment options, >60% of patients with TSC have treatment‐resistant epilepsy, 11 which can contribute to neurodevelopmental disabilities, including autism spectrum disorder and poor cognitive development, as well as status epilepticus and sudden unexpected death in epilepsy. 9 , 10 , 13 , 14 , 15 , 16
Highly purified add‐on cannabidiol (CBD) is approved as Epidiolex in the United States for treatment of seizures associated with Lennox–Gastaut syndrome (LGS), Dravet syndrome (DS), or TSC in patients ≥1 year of age and as Epidyolex in the UK and European Union in conjunction with clobazam for LGS and DS in patients ≥2 years of age; it is also approved for TSC in patients ≥2 years of age in the UK and the European Union. 17 , 18 In patients with medication‐resistant epilepsy associated with TSC, add‐on CBD produced significantly greater reduction than placebo in the number of TSC‐associated seizures and had an acceptable safety profile in a 16‐week (4‐week titration and 12‐week maintenance period), randomized, double‐blind, placebo‐controlled, multicenter phase 3 trial (GWPCARE6). 19 The effect of CBD treatment has been shown to start early—within 2 weeks of starting treatment—in clinical trials of patients with LGS and DS. 20 , 21 To assess whether early onset of treatment effect was also observed in patients with TSC, we used the data from GWPCARE6 to evaluate by‐day cumulative seizure reduction and incidence of adverse events (AEs), starting from the first day of titration. This analysis provides a more specific assessment of the timing of onset of the CBD treatment effect in terms of both efficacy and AEs.
2. MATERIALS AND METHODS
This was a post hoc analysis of an international, double‐blind, randomized, parallel‐group phase 3 trial of add‐on CBD versus placebo in patients with TSC and drug‐resistant epilepsy (GWPCARE6; NCT02544763). Trial design and patient eligibility criteria were published previously. 19 Briefly, the trial comprised a 4‐week baseline period followed by a 16‐week treatment time, which included 4 weeks of dose escalation (titration period) and 12 weeks of stable dosing (maintenance period). At the end of the treatment period, dosage was tapered over 10 days, followed by a 4‐week safety follow‐up. Patients who completed the blinded phase of the trial could continue to the open‐label extension phase. 22 Patients were eligible to enroll if they were 1–65 years old, had a definite clinical diagnosis of TSC with drug‐resistant epilepsy, had at least eight TSC‐associated seizures during the 4‐week baseline period with at least one seizure occurring in at least 3 of the 4 weeks, and were taking at least one ASM. One important exclusionary criterion was the use of an mTOR inhibitor such as everolimus or sirolimus.
Eligible patients were randomly assigned to receive a pharmaceutical formulation of highly purified CBD derived from Cannabis sativa L. (100 mg/ml oral solution; Epidiolex in the United States; Epidyolex in the UK, Northern Ireland, European Union, and Australia; GW Research, Cambridge, UK) at 25 mg/kg/day (CBD25) or 50 mg/kg/day (CBD50) or matched placebo. Trial medication was taken twice daily in equally divided doses. Patients started treatment at 5 mg/kg/day with the dose increasing by 5 mg/kg every 2 days until it reached 25 mg/kg/day on Day 9 for patients in both CBD dose groups. Dose escalation then slowed to an increase of 2.5 mg/kg every other day until the final maximum dose of 50 mg/kg/day was reached on Day 29 for patients in the CBD50 dose group only (Figure S1).
Patients or their caregivers recorded the number and type of seizures daily using an interactive voice‐response system and recorded AEs and concomitant medications using a paper diary. Efficacy was evaluated as the change from baseline in the number of TSC‐associated seizures in patients taking CBD versus placebo during the 16‐week treatment period. For this trial, TSC‐associated seizures were defined as countable focal motor seizures without impairment of awareness, focal seizures with impairment of awareness, focal seizures evolving to bilateral motor seizures, and generalized seizures (tonic–clonic, tonic, clonic, or atonic). This functional definition of TSC‐associated seizures was reviewed and approved by the US Food and Drug Administration, the European Medicines Agency, and the Epilepsy Study Consortium independent committee of experts. TSC‐associated seizures did not include absence, myoclonic, and focal sensory seizures, and infantile/epileptic spasms. On average, 94% of patients' seizures at baseline of this trial were TSC‐associated seizures. 19
Negative binomial regression on the sum of the seizure counts during the treatment period was used to evaluate the change from baseline in TSC‐associated seizures during the titration period and for Weeks 1–4, 5–8, and 9–12 of the maintenance period, and has been described previously. 19 Here, we report results of the analyses evaluating a more precise timing (by day) of onset of CBD antiseizure effect. Negative binomial regression analysis was used to calculate the percentage reduction in cumulative TSC‐associated seizure count for each day (i.e., including all previous treatment days) of the treatment period, starting with Day 1, for both doses of CBD and placebo. Percentage of patients with ≥50% reduction from baseline in the number of TSC‐associated seizures during the treatment period by cumulative day was also measured. Efficacy outcomes (percentage reduction in TSC‐associated seizures and ≥50% responder rate) are also presented for pooled CBD25 and CBD50 treatment groups for up to Day 11 of the titration period, when both groups were at 25‐mg/kg/day dosage. Nominal p values for comparison between placebo and pooled CBD groups were evaluated for each cumulative day; however, to avoid multiplicity associated with post hoc analyses, the nominal p value is reported only for the reduction in TSC‐associated seizures with CBD versus placebo. All randomized patients who received at least one dose of trial medication and had postbaseline efficacy data were included in the analyses.
To explore the effect of CBD using a measure that also accounts for the severity of the different seizure types, we evaluated the percentage reduction in composite focal seizure score by cumulative days. For calculation of composite focal seizure score, the focal seizure types were weighted based on the severity; focal seizures with impairment of awareness and focal seizures evolving to bilateral convulsive seizures were weighted greater than the focal motor seizures without impairment of awareness, as they are more likely to be associated with accidental trauma and sudden unexpected death in epilepsy. 23 Composite focal seizure score was then calculated as the sum of (1 × the number of focal motor seizures without impairment of awareness), (2 × the number of focal seizures with impairment of awareness), and (3 × the number of focal seizures evolving to bilateral convulsive seizures).
To assess the timing of treatment‐emergent AEs, we evaluated incidence of AEs during the 4‐week titration period and for Weeks 1–4, 5–8, and 9–12 of the maintenance period. The time to first onset of an AE was calculated as the start date of an AE minus the date of the first dose of CBD or placebo plus 1. Time to first event analysis by day was also conducted for the most frequent AEs of special interest. Time to resolution of AEs was categorized as the incidence of AEs that resolved within 4 weeks of onset, resolved >4 weeks after onset, or were ongoing at the end of treatment. The time to AE resolution was calculated as the stop date of an AE minus the start date of an AE plus 1. All patients who received at least one dose of trial medication were included in the safety analysis set. Samples to evaluate changes in the levels of liver enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were collected on Day 1 (before first dose of trial medication), on Days 15, 29, 43, 57, and 85, and at the end of treatment. This trial was conducted with Epidiolex/Epidyolex, and results do not apply to other CBD‐containing products.
3. RESULTS
3.1. Patients
Of 224 patients enrolled in the trial, 75 were randomly assigned to CBD25, 73 to CBD50, and 76 to placebo. At the end of treatment, 65 patients (87%) on CBD25 and 39 (53%) on CBD 50 reached or remained at their assigned dosage. Twenty‐three patients (10 [13%] on CBD25, 12 [16%] on CBD50, and one [1%] on placebo) withdrew from the trial, with AEs as the most common primary reason for withdrawal (eight patients [11%] taking CBD25, eight [11%] taking CBD50, and none taking placebo). Overall, 201 patients (65 [87%] taking CBD25, 61 [84%] taking CBD50, and 75 [99%] taking placebo) completed the treatment, and 199 of these patients (99.0%) continued to the open‐label extension phase.
Baseline characteristics were similar between the treatment groups (Table 1). The median (range) age of patients was 11.3 years (1.1–56.8), and 74% of patients were <18 years of age. Most of the patients were male (58%), White (90%), and were taking a median (range) of 3 (0–5) ASMs at baseline and had tried and discontinued 4 (0–15); the most frequently used ASMs at baseline were valproate (45%), vigabatrin (33%), levetiracetam (29%), and clobazam (27%). Patients had a median (first quartile, third quartile) of 57 (28, 107) TSC‐associated seizures during the 4‐week baseline period.
TABLE 1.
Demographics and clinical characteristics of patients at baseline
| Characteristic | Placebo, n = 76 | CBD25, n = 75 | CBD50, n = 73 |
|---|---|---|---|
| Age, years, median (range) | 10.9 (1.2–55.8) | 11.6 (1.1–56.8) | 10.2 (1.8–34.9) |
| Sex, n (%) | |||
| Female | 31 (41) | 32 (43) | 30 (41) |
| Male | 45 (59) | 43 (57) | 43 (59) |
| Number of ASMs, median (range) | |||
| Prior | 4 (0–15) | 4 (0–13) | 4 (0–13) |
| Current a | 3 (1–5) | 3 (0–4) | 3 (1–5) |
| Current ASMs [>20%], n (%) | |||
| Valproate | 35 (46) | 29 (39) | 36 (49) |
| Vigabatrin | 17 (22) | 28 (37) | 29 (40) |
| Levetiracetam | 24 (32) | 19 (25) | 22 (30) |
| Clobazam | 25 (33) | 17 (23) | 19 (26) |
| Number of TSC‐associated seizures during the 28‐day baseline period, median (Q1, Q3) b | 54 (26, 102) | 56 (21, 101) | 61 (36, 117) |
| Composite focal seizure score during the 28‐day baseline period, median (Q1, Q3) c | 75 (33, 164) | 87 (37, 190) | 92 (49, 217) |
| Focal seizure types, n (%) | |||
| Focal seizures without impaired awareness | 33 (43) | 29 (39) | 39 (53) |
| Focal seizures with impaired awareness | 50 (66) | 46 (61) | 54 (74) |
| Focal to bilateral motor seizures | 24 (32) | 17 (23) | 24 (33) |
Abbreviations: ASM, antiseizure medication; CBD25, cannabidiol 25 mg/kg/day; CBD50, cannabidiol 50 mg/kg/day; Q1, first quartile; Q3, third quartile; TSC, tuberous sclerosis complex.
One patient was on no concomitant medications during the trial. This patient had previously discontinued five other ASMs, with the last one stopped 6 months prior to enrolling in GWPCARE6. At the time of enrollment, the protocol did not explicitly state that patients had to be taking at least one ASM, but the protocol was subsequently amended to clarify this.
TSC‐associated seizures for this trial were defined as countable focal motor seizures without impairment of awareness, focal seizures with impairment of awareness, focal seizures evolving to bilateral motor seizures, and generalized seizures (tonic–clonic, tonic, clonic, or atonic); they did not include absence, myoclonic, and focal sensory seizures, and infantile/epileptic spasms.
Composite focal seizure score was calculated as the sum of (1 × the number of focal motor seizures without impairment of awareness), (2 × the number of focal seizures with impairment of awareness), and (3 × the number of focal seizures evolving to bilateral convulsive seizures).
3.2. Efficacy
A greater percentage reduction in the number of TSC‐associated seizures with CBD than placebo was first observed during the titration period, with 37% (95% confidence interval [CI] = 28%–44%) reduction from baseline observed for CBD25, 36% (95% CI = 27%–43%) for CBD50, and 18% (95% CI = 8%–27%) for placebo during the 4‐week titration period. 19 In the analysis by cumulative days, difference in seizure reduction between placebo and CBD emerged by Day 6 (at 15 mg/kg/day) and reached significance by Day 10 (at 25 mg/kg/day, nominal p = .049); the reduction in seizure count was maintained throughout the treatment period (Figure 1A). More patients taking CBD had ≥50% reduction in TSC‐associated seizure count than those taking placebo, and the difference emerged as early as Day 10 of the titration period and was maintained throughout the treatment period (Figure 1B).
FIGURE 1.
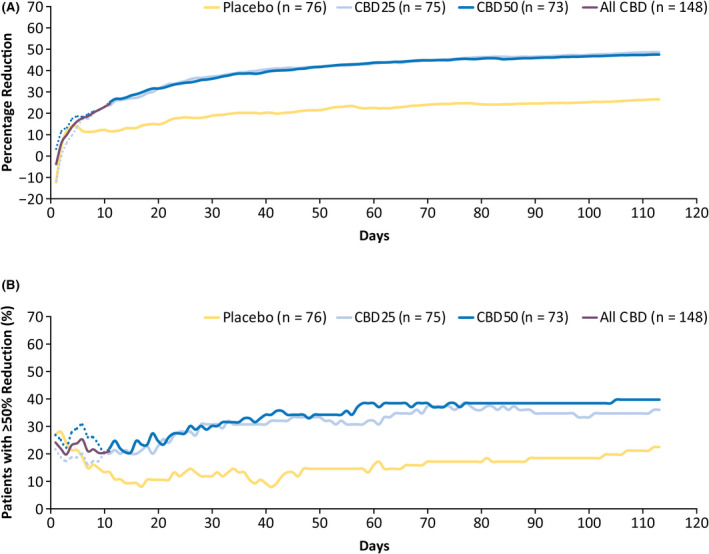
Efficacy outcomes. (A) Cumulative percentage reduction from baseline in the number of tuberous sclerosis complex (TSC)‐associated seizures by day and (B) percentage of patients with ≥50% reduction in TSC‐associated seizures by day. Primary endpoint seizures included all countable focal motor seizures without impairment of awareness, focal seizures with impairment of awareness, focal seizures evolving to bilateral motor seizures, and generalized seizures (tonic–clonic, tonic, clonic, or atonic); they excluded absence, myoclonic, focal sensory, and infantile/epileptic spasms. The dotted line for each dose group represents the data until Day 11, when all patients were receiving the same dose of the trial drug, reflecting small variations in response not attributable to difference in dosage. All CBD, represented by the purple line, shows combined data for both CBD groups up to Day 11, after which patients in the CBD25 dose group remained on 25 mg/kg/day and those in the CBD50 dose group continued titration up to 50 mg/kg/day. CBD, cannabidiol; CBD25, cannabidiol 25 mg/kg/day; CBD50, cannabidiol 50 mg/kg/day; TSC, tuberous sclerosis complex
The early onset of the CBD antiseizure effect was also observed for the composite focal seizure score, with the difference between CBD and placebo emerging during the first week of titration (Figure 2). By the end of the treatment period, percentage reductions (95% CI) in composite focal seizure scores were 52% (42%–60%) for CBD25, 50% (41%–58%) for CBD50, and 32% (20%–43%) for placebo.
FIGURE 2.
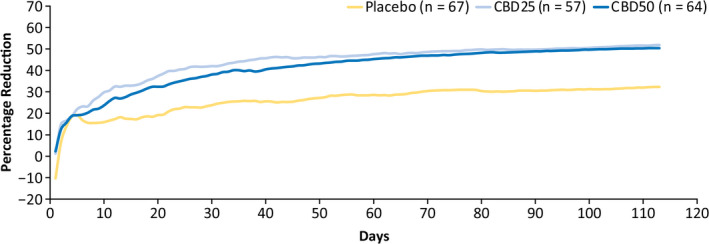
Cumulative percentage reduction from baseline in focal composite seizure score by day. Composite focal seizure score was calculated as the sum of (1 × the number of focal motor seizures without impairment of awareness), (2 × the number of focal seizures with impairment of awareness), and (3 × the number of focal seizures evolving to bilateral convulsive seizures). CBD25, cannabidiol 25 mg/kg/day; CBD50, cannabidiol 50 mg/kg/day
3.3. Safety
Treatment‐emergent AEs were reported in 70 patients (93%) taking CBD25, 73 patients (100%) taking CBD50, and 72 patients (95%) taking placebo. Eighteen patients (12%) taking CBD permanently discontinued treatment because of an AE compared with two patients (3%) on placebo. The most common AEs leading to discontinuation were rash (four patients [3%] in the CBD group) and ALT level elevations, somnolence, and urticaria (two patients [1%] each in the CBD group). There were no deaths during the trial. The most frequently reported AEs were diarrhea in 43% of patients taking CBD versus 25% taking placebo, decreased appetite in 22% of patients taking CBD versus 12% taking placebo, and somnolence in 20% of patients taking CBD versus 9% taking placebo.
Most patients first reported an AE during the titration period, with 64% of patients taking CBD and 54% taking placebo reporting an AE within 2 weeks of starting treatment (Figure 3). The number of patients reporting onset of an AE decreased as treatment progressed (Figure 3). In the time‐to‐first‐event analysis, clear separation in onset of diarrhea between placebo and CBD emerged by Day 10 of titration, when patients were at the 25‐mg/kg/day dosage (Figure 4A). Most patients reported the first occurrence of decreased appetite (Figure 4B) within 40 days and the first occurrence of somnolence or sedation (Figure 4C) within 20 days of starting CBD or placebo treatment.
FIGURE 3.
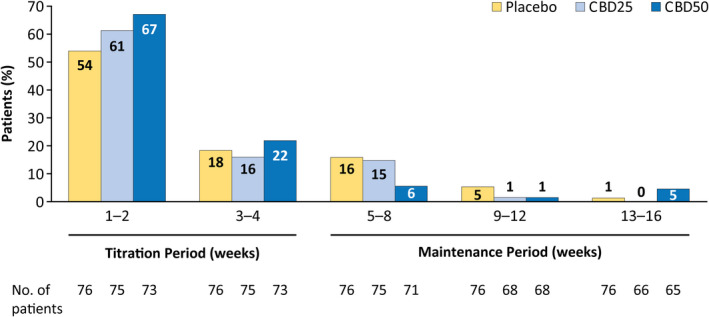
Adverse events by time of onset. If a patient had more than one occurrence of an AE, only the first occurrence of that AE was counted. The percentages of patients are based on the number of patients in the safety analysis set who had a visit or follow‐up call within each time period. AE, adverse event; CBD25, cannabidiol 25 mg/kg/day; CBD50, cannabidiol 50 mg/kg/day
FIGURE 4.
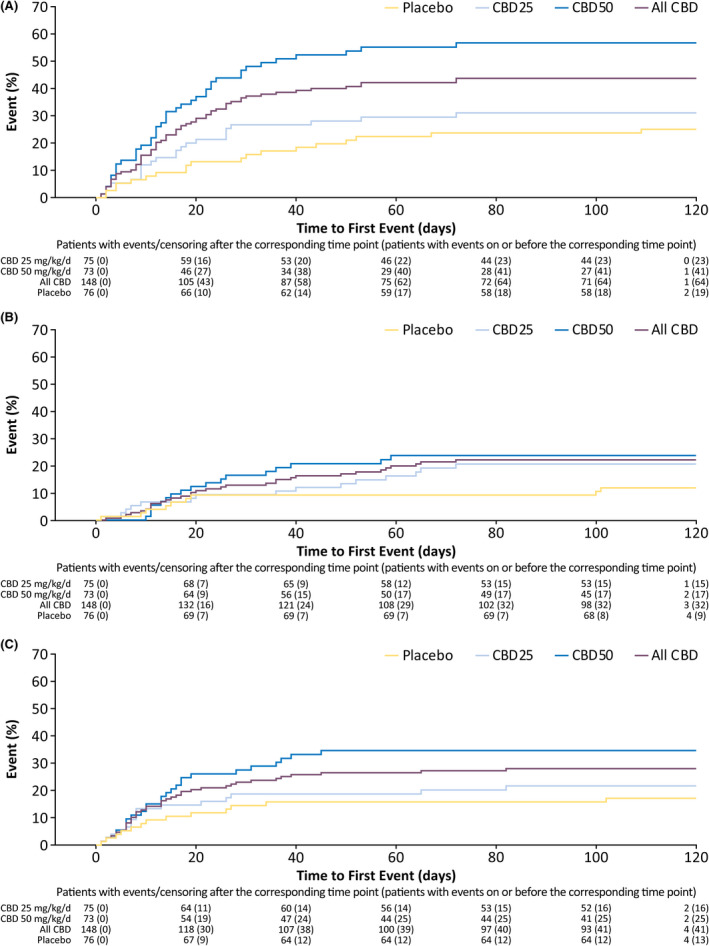
Time to first occurrence of the three most common adverse events: (A) diarrhea, (B) decreased appetite, and (C) somnolence or sedation. Somnolence and sedation also include the preferred terms fatigue, lethargy, asthenia, and malaise. CBD, cannabidiol; CBD25, cannabidiol 25 mg/kg/day; CBD50, cannabidiol 50 mg/kg/day
Overall, AEs resolved within 4 weeks of onset in 30% of patients and before the end of the 16‐week trial in 58% of patients (Figure 5). Of the patients reporting the most common AEs of diarrhea, decreased appetite, and somnolence, events resolved within 4 weeks in 41%–64% of patients and by the end of the trial in 69%–88% of patients (Figure 5).
FIGURE 5.
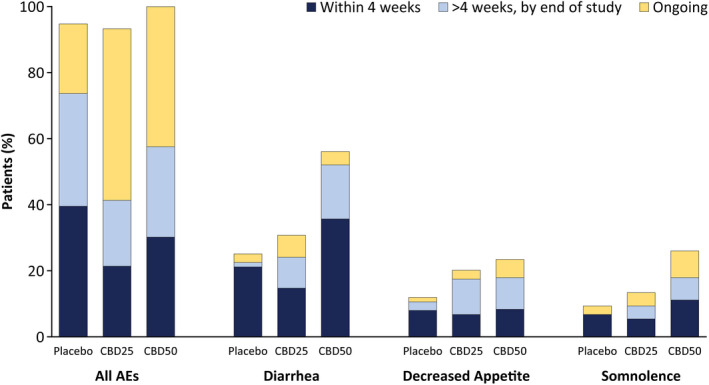
Occurrence of AEs by time to AE resolution. For patients who had multiple occurrences of an AE, only one occurrence with the longest time to resolution was counted. Any AEs that did not resolve were counted once as ongoing. AE, adverse event; CBD25, cannabidiol 25 mg/kg/day; CBD50, cannabidiol 50 mg/kg/day
On the basis of laboratory investigation of liver transaminase levels, elevation in ALT or AST (>3× upper limit of normal) occurred in nine of 75 (12%) patients taking CBD25 and 19 of 73 (26%) patients taking CBD50; no patient taking placebo had increased transaminase levels >3× upper limit of normal (Table S1). Of the 28 patients with elevations, 22 (79%) were on concomitant valproate. Elevations in most patients (67% of patients taking CBD25 and 74% taking CBD50) occurred within 30 days of starting treatment. No patient met the standard criteria for severe drug‐induced liver injury (Hy's law). Elevated ALT/AST levels resolved in all patients: spontaneously in 13 patients, after discontinuation from trial in five patients, and after CBD or ASM dose reduction in 10 patients.
4. DISCUSSION
This post hoc analysis of efficacy and safety data from a randomized placebo‐controlled trial of add‐on CBD in patients with TSC was conducted to evaluate a more precise timing of the CBD treatment effect. Difference in seizure reduction between placebo and CBD emerged by Day 6 (both CBD dose groups at 15 mg/kg/day) and reached nominal significance by Day 10 (both groups at 25 mg/kg/day); difference in ≥50% responder rates was also observed by Day 10. Thus, our results suggest that the CBD treatment effect may occur as early as within 6–10 days of titration and at doses lower than 25 mg/kg/day. However, the rapid uptitration makes it difficult to ascertain what the response would have been if patients had stayed on a dose <25 mg/kg/day.
The early onset of antiseizure effect is important because a faster onset can improve patient perception, potentially leading to better treatment adherence, may reduce the risk of preventable seizure‐related injuries more quickly after treatment initiation, and allows for a faster decision on whether to continue or switch treatments. However, percentage reduction in seizure count and the proportion of ≥50% responders continued to increase over the full treatment period of the trial, suggesting that some patients who do not have an early response may still benefit later during the treatment.
ASMs are the most common treatment for seizures associated with TSC; of the patients who receive treatment for epilepsy, >99% have been prescribed ASMs, and more than half of these patients have tried at least three distinct ASMs. 24 However, limited data are available for time to onset of treatment effect for most ASMs in TSC. For the mTOR pathway inhibitor everolimus, time‐dependent increase in seizure reduction was observed, with longer exposure needed for greater antiseizure effect. 25 , 26 The antiseizure effect of vigabatrin, one of the most frequently used medications for TSC‐associated seizures, 27 has been observed within 1 week of starting treatment in children with infantile spasms with or without TSC. 28 , 29 Both vigabatrin 30 and CBD 31 reach steady state after approximately 2 days, supporting their early onset of treatment effect. The early onset of CBD's treatment effect in terms of efficacy was also observed in clinical trials of patients with LGS and DS, 20 , 21 during which a significant difference in seizure reduction between placebo and CBD was observed within 2 weeks of starting treatment, demonstrating a consistent effect of CBD treatment across several treatment‐resistant epilepsies.
Similar to efficacy results, timing for onset of AEs also supports a relatively fast CBD treatment effect. More than 60% of patients receiving CBD reported AEs within 2 weeks of starting treatment. Most patients who experienced diarrhea, decreased appetite, and somnolence (the most frequently reported AEs with CBD) had the first incidence during the titration period. The most common AEs—diarrhea, decreased appetite, and somnolence—typically resolved during the trial (>68% of cases) and often within 4 weeks (>40% of cases). Early onset and resolution of AEs suggest that some AEs occurred early when patients were adapting to treatment and resolved as patients became habituated. Additionally, it is possible that because the AEs that occurred in most patients (88%) were of mild or moderate severity, 19 they were less likely to be reported later during the trial.
A dose‐dependent increase in levels of ALT/AST was observed in 12% of patients taking CBD25 and in 26% of patients taking CBD50 during the trial and tended to occur early in treatment. Consistent with prior reports, 32 , 33 , 34 , 35 most patients (79%) with elevation in transaminase levels in this trial were also taking concomitant valproate. Although precise timing for the elevations could not be determined because the testing did not start until Day 15, 71% of patients with an elevation experienced it during the first 30 days of treatment. All cases of ALT/AST elevations resolved, spontaneously in most patients (46%), or after ASM dose reduction (36%) or treatment discontinuation (18%) in others.
Our study has limitations. Because this was a post hoc analysis, the interpretability and generalizability of the results are limited. Although a nominally significant difference was observed in the antiseizure effect of placebo and CBD during the early time points, it may not prove to be statistically significant in a prospectively designed trial. However, early treatment effect is supported by data from CBD trials in patients with LGS and DS. 20 , 21 The results of this analysis may differ from real‐world use and for individual patients. Compared with what is prescribed in the US label, 17 the initial dose escalation in this trial was faster, which may have led to a relatively faster response. In clinical practice, clinicians may need to individualize the titration schedule for patients to balance efficacy and AEs. A delayed response may also occur in some patients because of their daily seizure frequency or other disease characteristics.
5. CONCLUSIONS
Results of this post hoc analysis of data from GWPCARE6, a randomized, controlled phase 3 trial of add‐on CBD in patients with TSC, demonstrate that the antiseizure effect of CBD emerged within 6–10 days of starting treatment. Onset of AEs was most frequent during the first 2 weeks of the titration period, and the majority of AEs resolved during the 16‐week treatment period. Overall, our results suggest that the CBD treatment effect may occur within 2 weeks of starting treatment.
CONFLICT OF INTEREST
J.Y.W. has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Novartis Pharmaceuticals and GW Pharmaceuticals and has received research support from both. She has also received support from the National Institutes of Health and TSC Alliance. H.R.C. has received research support as a site investigator from GW Research and from Novartis during the conduct of the study; grant support from the National Institute of Neurological Disorders and Stroke (NINDS); nonfinancial support from the International League Against Epilepsy and European Academy of Neurology; and personal fees for consulting from Bial Pharma and in relation to medicolegal expert witness reports from civil court proceedings in the UK for personal injury cases (via solicitors). O.D. has equity interests in Qstate Biosciences, Tevard Biosciences, Regel Therapeutics, Script Biosciences, Privateer Holdings, Tilray, Receptor Life Sciences, Empatica, Engage, Egg Rock/Papa & Barkley, Rettco, SilverSpike, and California Cannabis Enterprises and receives grant support from NINDS, National Institute of Mental Health, Multidisciplinary University Research Initiative, US Centers for Disease Control and Prevention, and National Science Foundation. He is an investigator for PTC Therapeutics, Stoke Therapeutics, Marinus, Ovid, and GW Pharmaceuticals. C.J. has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Zogenix. I.M. has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with GW Pharmaceuticals, TS Alliance, DS Foundation, lnsys, Biomarin, Upsher‐Smith, Visualase, Neuroblate, Zogenix, and Ultragenyx. He has received personal compensation in an editorial capacity for Insys Pharmaceuticals, GW Pharmaceuticals, TS Alliance, DS Foundation, Visualase, Neuroblate, Zogenix, and Ultragenyx and has received research support from GW Pharmaceuticals, TS Alliance, DS Foundation, lnsys, Biomarin, Upsher‐Smith, Visualase, Neuroblate, Zogenix, and Ultragenyx. C.M.R. has nothing to disclose. R.S.‐C. has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with scientific advisory boards of GW Pharmaceuticals, Zogenix, and Novartis; is a member of the speaker's bureau of GW Pharmaceuticals; and has received research support as a principal investigator for GW Pharmaceuticals‐, Zogenix‐, and Takeda‐initiated trials. D.C. is an employee of GW Research. F.S. is an employee of Greenwich Biosciences. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
AUTHOR CONTRIBUTIONS
All authors provided substantial contributions to the conception or design of the work or the acquisition, analysis, or interpretation of data for the work; drafted the work or revised it critically for important intellectual content; and provided final approval of the version to be published.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the patients, their families, and the staff at sites that participated in this trial. Medical writing support for the development of this article, under the direction of the authors, was provided by Ritu Pathak, PhD, and editing support by Dena McWain, both of Ashfield MedComms, an Ashfield Health company, and funded by Greenwich Biosciences.
Wu JY, Cock HR, Devinsky O, Joshi C, Miller I, Roberts CM, et al. Time to onset of cannabidiol treatment effect and resolution of adverse events in tuberous sclerosis complex: Post hoc analysis of randomized controlled phase 3 trial GWPCARE6. Epilepsia. 2022;63:1189–1199. doi: 10.1111/epi.17199
Joyce Y. Wu was affiliated with UCLA Mattel Children's Hospital, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California, USA, at the time this trial was conducted.
Ian Miller was affiliated with Nicklaus Children's Hospital, Miami, Florida, USA, at the time this trial was conducted.
Funding information
This trial was sponsored by GW Research, Cambridge, UK.
REFERENCES
- 1. Northrup H, Krueger DA, International Tuberous Sclerosis Complex Consensus Group . Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2012;2013(49):243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372:657–68. [DOI] [PubMed] [Google Scholar]
- 3. Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–56. [DOI] [PubMed] [Google Scholar]
- 4. Chan JA, Zhang H, Roberts PS, Jozwiak S, Wieslawa G, Lewin‐Kowalik J, et al. Pathogenesis of tuberous sclerosis subependymal giant cell astrocytomas: biallelic inactivation of TSC1 or TSC2 leads to mTOR activation. J Neuropathol Exp Neurol. 2004;63:1236–42. [DOI] [PubMed] [Google Scholar]
- 5. Wang S, Fallah A. Optimal management of seizures associated with tuberous sclerosis complex: current and emerging options. Neuropsychiatr Dis Treat. 2014;10:2021–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tuberous Sclerosis Alliance . Diagnosis, surveillance, and management for healthcare professionals. Cited 2021 Sep 14. Available from: https://www.tsalliance.org/healthcare‐professionals/diagnosis/
- 7. Kingswood JC, d'Augères GB, Belousova E, Ferreira JC, Carter T, Castellana R, et al. TuberOus SClerosis registry to increase disease Awareness (TOSCA)—baseline data on 2093 patients. Orphanet J Rare Dis. 2017;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jeong A, Wong M. Systemic disease manifestations associated with epilepsy in tuberous sclerosis complex. Epilepsia. 2016;57:1443–9. [DOI] [PubMed] [Google Scholar]
- 9. de Vries PJ, Wilde L, de Vries MC, Moavero R, Pearson DA, Curatolo P. A clinical update on tuberous sclerosis complex‐associated neuropsychiatric disorders (TAND). Am J Med Genet C Semin Med Genet. 2018;178:309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Vries PJ, Belousova E, Benedik MP, Carter T, Cottin V, Curatolo P, et al. TSC‐associated neuropsychiatric disorders (TAND): findings from the TOSCA natural history study. Orphanet J Rare Dis. 2018;13:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chu‐Shore CJ, Major P, Camposano S, Muzykewicz D, Thiele EA. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia. 2010;51:1236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Specchio N, Pietrafusa N, Trivisano M, Moavero R, De Palma L, Ferretti A, et al. Autism and epilepsy in patients with tuberous sclerosis complex. Front Neurol. 2020;11:639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Curatolo P, Nabbout R, Lagae L, Aronica E, Ferreira JC, Feucht M, et al. Management of epilepsy associated with tuberous sclerosis complex: updated clinical recommendations. Eur J Paediatr Neurol. 2018;22:738–48. [DOI] [PubMed] [Google Scholar]
- 14. Amin S, Lux A, Calder N, Laugharne M, Osborne J, O'Callaghan F. Causes of mortality in individuals with tuberous sclerosis complex. Dev Med Child Neurol. 2017;59:612–7. [DOI] [PubMed] [Google Scholar]
- 15. Overwater IE, Verhaar BJ, Lingsma HF, Bindels‐de Heus GC, van den Ouweland AM, Nellist M, et al. Interdependence of clinical factors predicting cognition in children with tuberous sclerosis complex. J Neurol. 2017;264:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Capal JK, Bernardino‐Cuesta B, Horn PS, Murray D, Byars AW, Bing NM, et al. Influence of seizures on early development in tuberous sclerosis complex. Epilepsy Behav. 2017;70:245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Epidiolex® (cannabidiol) oral solution [prescribing information]. Carlsbad, CA: Greenwich Biosciences. 2020. Oct [cited 2020 Nov 18]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/210365s008lbl.pdf [Google Scholar]
- 18. Epidyolex® (cannabidiol) oral solution [summary of product characteristics]. Amersfoort, the Netherlands: GW Pharma (International). 2021. [cited 2021 Dec 10]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/epidyolex#product‐information‐section [Google Scholar]
- 19. Thiele EA, Bebin EM, Bhathal H, Jansen FE, Kotulska K, Lawson JA, et al. Add‐on cannabidiol treatment for drug‐resistant seizures in tuberous sclerosis complex: a placebo‐controlled randomized clinical trial. JAMA Neurol. 2021;78:285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Privitera M, Bhathal H, Wong M, Cross JH, Wirrell E, Marsh ED, et al. Time to onset of cannabidiol (CBD) treatment effect in Lennox‐Gastaut syndrome: analysis from two randomized controlled trials. Epilepsia. 2021;62:1130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Madan Cohen J, Checketts D, Dunayevich E, Gunning B, Hyslop A, Madhavan D, et al. Time to onset of cannabidiol treatment effects in Dravet syndrome: analysis from two randomized controlled trials. Epilepsia. 2021;62:2218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thiele EA, Bebin EM, Filloux F, Kwan P, Loftus R, Sahebkar F, et al. Long‐term cannabidiol treatment for seizures in patients with tuberous sclerosis complex: an open‐label extension trial. Epilepsia. 2021;63:426–39. https://doi.org/ 10.1111/epi.17150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nashef L, Hindocha N, Makoff A. Risk factors in sudden death in epilepsy (SUDEP): the quest for mechanisms. Epilepsia. 2007;48:859–71. [DOI] [PubMed] [Google Scholar]
- 24. Song J, Swallow E, Said Q, Peeples M, Meiselbach M, Signorovitch J, et al. Epilepsy treatment patterns among patients with tuberous sclerosis complex. J Neurol Sci. 2018;391:104–8. [DOI] [PubMed] [Google Scholar]
- 25. Krueger DA, Wilfong AA, Holland‐Bouley K, Anderson AE, Agricola K, Tudor C, et al. Everolimus treatment of refractory epilepsy in tuberous sclerosis complex. Ann Neurol. 2013;74:679–87. [DOI] [PubMed] [Google Scholar]
- 26. French JA, Lawson JA, Yapici Z, Ikeda H, Polster T, Nabbout R, et al. Adjunctive everolimus therapy for treatment‐resistant focal‐onset seizures associated with tuberous sclerosis (EXIST‐3): a phase 3, randomised, double‐blind, placebo‐controlled study. Lancet. 2016;388:2153–63. [DOI] [PubMed] [Google Scholar]
- 27. Overwater IE, Bindels‐de Heus K, Rietman AB, Ten Hoopen LW, Vergouwe Y, Moll HA, et al. Epilepsy in children with tuberous sclerosis complex: chance of remission and response to antiepileptic drugs. Epilepsia. 2015;56:1239–45. [DOI] [PubMed] [Google Scholar]
- 28. Curatolo P, Verdecchia M, Bombardieri R. Vigabatrin for tuberous sclerosis complex. Brain Dev. 2001;23:649–53. [DOI] [PubMed] [Google Scholar]
- 29. Hancock E, Osborne JP. Vigabatrin in the treatment of infantile spasms in tuberous sclerosis: literature review. J Child Neurol. 1999;14:71–4. [DOI] [PubMed] [Google Scholar]
- 30. Hoke JF, Yuh L, Antony KK, Okerholm RA, Elberfeld JM, Sussman NM. Pharmacokinetics of vigabatrin following single and multiple oral doses in normal volunteers. J Clin Pharmacol. 1993;33:458–62. [DOI] [PubMed] [Google Scholar]
- 31. Taylor L, Gidal B, Blakey G, Tayo B, Morrison G. A phase I, randomized, double‐blind, placebo‐controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs. 2018;32:1053–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thiele EA, Marsh ED, French JA, Mazurkiewicz‐Beldzinska M, Benbadis SR, Joshi C, et al. Cannabidiol in patients with seizures associated with Lennox‐Gastaut syndrome (GWPCARE4): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet. 2018;391:1085–96. [DOI] [PubMed] [Google Scholar]
- 33. Miller I, Scheffer IE, Gunning B, Sanchez‐Carpintero R, Gil‐Nagel A, Perry MS, et al. Dose‐ranging effect of adjunctive oral cannabidiol vs placebo on convulsive seizure frequency in Dravet syndrome: a randomized clinical trial. JAMA Neurol. 2020;77:613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, et al. Effect of cannabidiol on drop seizures in the Lennox‐Gastaut syndrome. N Engl J Med. 2018;378:1888–97. [DOI] [PubMed] [Google Scholar]
- 35. Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, et al. Trial of cannabidiol for drug‐resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


