Abstract
Aim
To assess selected cardiorenal outcomes with ertugliflozin according to use of baseline glucose‐lowering agent.
Materials and Methods
VERTIS CV was a cardiovascular (CV) outcome trial for ertugliflozin versus placebo, conducted in patients with type 2 diabetes and established atherosclerotic CV disease. The primary outcome was time to the first event of CV death, myocardial infarction or stroke (major adverse CV events [MACE]), with other CV outcomes also assessed. Outcomes were analysed using Cox proportional hazards models stratified by baseline use of metformin, insulin, sulphonylureas (SUs) and dipeptidyl peptidase‐4 (DPP‐4) inhibitors, with interaction testing to assess for treatment effect modification. Changes from baseline in glycaemic, metabolic and haemodynamic variables were also assessed.
Results
Of 8246 randomized patients, at baseline 6286 (76%) were on metformin, 3898 (47%) were on insulin, 3383 (41%) were on SUs and 911 (11%) were on DPP‐4 inhibitors, alone or in combination therapy (67% used >1 glucose‐lowering agent at baseline). For each glucose‐lowering agent evaluated, no evidence for effect modification was observed for MACE by baseline use of metformin (with: hazard ratio [HR] 0.92, 95% confidence interval [CI] 0.790, 1.073; without: 1.13, 95% CI 0.867, 1.480), insulin (with: HR 0.91, 95% CI 0.765, 1.092; without: 1.06, 95% CI 0.867, 1.293), SUs (with: HR 1.11, 95% CI 0.890, 1.388; without: 0.90, 95% CI 0.761, 1.060) or DPP‐4 inhibitors (with: HR 0.77, 95% CI 0.502, 1.173; without: 1.00, 95% CI 0.867, 1.147) (all P interaction > 0.05). Similar results were observed for all secondary outcomes analysed.
Conclusion
In VERTIS CV, the effects of ertugliflozin on cardiorenal outcomes were consistent across subgroups of patients stratified by baseline glucose‐lowering agent.
ClinicalTrials.gov identifier: NCT01986881
Keywords: cardiovascular disease, SGLT2 inhibitor, type 2 diabetes
1. INTRODUCTION
Patients with type 2 diabetes mellitus (T2DM) often require combination therapy with oral glucose‐lowering agents due to disease progression. 1 Sodium‐glucose cotransporter 2 (SGLT2) inhibitors are effective as add‐on treatment for patients with T2DM inadequately controlled on glucose‐lowering agents. SGLT2 inhibitors are not associated with hypoglycaemia when administered as monotherapy or in combination with other agents that by themselves do not cause hypoglycaemia. 2 In patients with T2DM, SGLT2 inhibitors also provide modest reductions in body weight and blood pressure. 3 In addition to providing glycaemic control, large cardiovascular (CV) outcome trials (CVOTs) have demonstrated that SGLT2 inhibitors reduce the risk of CV events, including hospitalization for heart failure (HHF), and preserve kidney function. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 The majority of patients with T2DM included in CVOTs had been taking at least one glucose‐lowering agent at baseline, with up to 82% of patients on metformin, 50% on insulin, 43% on sulphonylureas (SUs) and 17% on dipeptidyl peptidase‐4 (DPP‐4) inhibitors across CVOTs with SGLT2 inhibitors. 4 , 5 , 6 , 10 Given the high proportion of patients on glucose‐lowering agents at baseline in these studies, it is of interest to explore whether cardiorenal outcomes of SGLT2 inhibitors are influenced by background antihyperglycaemic treatment.
VERTIS CV was a CVOT for the SGLT2 inhibitor ertugliflozin in patients with T2DM and atherosclerotic CV disease (ASCVD). 10 These post hoc analyses evaluated selected CV and kidney outcomes in patients with T2DM and ASCVD treated with different glucose‐lowering agents at enrolment in the VERTIS CV trial.
2. MATERIALS AND METHODS
2.1. Study overview
VERTIS CV (ClinicalTrials.gov identifier: NCT01986881) was a multicentre, randomized, double‐blind, placebo‐controlled, parallel‐group, event‐driven study. 10 , 12 Participants were eligible if they were aged ≥40 years with T2DM (glycated haemoglobin [HbA1c] 53‐91 mmol/mol [7.0%‐10.5%], inclusive), and had stable, established ASCVD involving the coronary, cerebrovascular and/or peripheral arterial systems. The VERTIS CV trial was conducted in compliance with the ethical principles of the Declaration of Helsinki and in compliance with all International Conference on Harmonisation Good Clinical Practice Guidelines. The final protocol and informed consent documentation were reviewed and approved by the institutional review board or independent ethics committee at each investigational centre. Written informed consent was obtained from all participants. Patients were randomly assigned (1:1:1) to oral, once‐daily ertugliflozin 5 mg, 15 mg or placebo.
2.2. Assessment of outcomes
In these analyses, patients were stratified by use of selected glucose‐lowering agents at baseline. The primary outcome was major adverse CV events (MACE), a composite of death from CV causes, nonfatal myocardial infarction or nonfatal stroke. The secondary outcomes were: a composite of death from CV causes or HHF; death from CV causes; a composite of death from kidney causes, kidney replacement therapy or doubling of the serum creatinine level; and HHF. An additional exploratory efficacy outcome was a kidney‐specific composite outcome of a sustained decrease of 40% or more in estimated glomerular filtration rate (eGFR) to less than 60 mL/min/1.73 m2, new end‐stage kidney disease or death from kidney causes. Subgroup analyses were conducted according to baseline use (Yes/No) of glucose‐lowering agent (metformin, insulin, SUs and DPP‐4 inhibitors). An assessment of outcomes according to the number of glucose‐lowering agents used at baseline was also conducted. Changes from baseline in HbA1c, body weight, systolic blood pressure (SBP), eGFR and urine albumin to creatinine ratio (UACR) were also assessed.
2.3. Statistical analyses
Baseline characteristics are reported as frequencies and percentages for categorical variables and as mean and standard deviation (SD) for continuous variables. The data from the two ertugliflozin dose groups were prespecified to be pooled for the assessment of CV and kidney outcomes. For each baseline glucose‐lowering agent, the effect of ertugliflozin on the efficacy outcome was assessed using a Cox proportional hazards model including terms for treatment (all ertugliflozin vs. placebo), subgroup (use vs. non‐use of glucose‐lowering agent at baseline) and the interaction of treatment by subgroup. Analysis of the primary outcome was performed with data from all the patients who had undergone randomization and received at least one dose of ertugliflozin or placebo and included events that occurred up to 365 days after the confirmed last dose. The analyses of the secondary outcomes were performed on an intention‐to‐treat basis, using all patients who had undergone randomization, and all time on‐study for each patient. To test for treatment effect modification of baseline glucose‐lowering agent, hazard ratios (HRs) and 95% confidence intervals (CIs) were determined from the Cox proportional hazards model, along with the P value for the interaction of treatment by baseline use of glucose‐lowering agent.
3. RESULTS
3.1. Baseline characteristics
In VERTIS CV, a total of 8246 patients with T2DM and ASCVD underwent randomization; 8238 patients received at least one dose of ertugliflozin or placebo. 10 , 12 Of the 8246 patients randomized, at baseline 6286 (76%) used metformin, 3898 (47%) insulin, 3383 (41%) SUs and 911 (11%) DPP‐4 inhibitors, alone or in combination therapy. Baseline characteristics of patients by glucose‐lowering agent use are shown in Table 1. Some differences were observed when comparing baseline users versus non‐users of each glucose‐lowering agent class. Briefly, baseline eGFR levels were lower in insulin and SU users yet higher in metformin users compared with non‐users. Baseline UACR levels were lower in metformin users yet higher in insulin users compared with non‐users. Insulin users had a longer disease duration than non‐users. Of the metformin users, 18.3% were on a single glucose‐lowering agent at baseline (ie, metformin monotherapy). Overall, 67% of all VERTIS CV participants used more than one glucose‐lowering agent at baseline (Table 1).
TABLE 1.
Baseline characteristics by baseline glucose‐lowering agent use
| Characteristic | Baseline glucose‐lowering agent use | |||||||
|---|---|---|---|---|---|---|---|---|
| Metformin | Insulin | SUs | DPP‐4 inhibitors | |||||
| Yes n = 6286 | No n = 1960 | Yes n = 3898 | No n = 4348 | Yes n = 3383 | No n = 4863 | Yes n = 911 | No n = 7335 | |
| Age, years, mean (SD) | 64.0 (7.9) | 65.7 (8.4) | 64.6 (7.8) | 64.2 (8.3) | 64.5 (8.2) | 64.3 (8.0) | 65.0 (8.3) | 64.3 (8.0) |
| Male sex, n (%) | 4446 (70.7) | 1323 (67.5) | 2663 (68.3) | 3106 (71.4) | 2396 (70.8) | 3373 (69.4) | 704 (77.3) | 5065 (69.1) |
| BMI, kg/m2, mean (SD) a | 32.0 (5.4) | 31.8 (5.5) | 32.7 (5.4) | 31.3 (5.2) | 31.4 (5.2) | 32.3 (5.5) | 31.3 (5.5) | 32.0 (5.4) |
| White race, n (%) | 5506 (87.6) | 1734 (88.5) | 3427 (87.9) | 3813 (87.7) | 2931 (86.6) | 4309 (88.6) | 753 (82.7) | 6487 (88.4) |
| Region, n (%) | ||||||||
| Europe | 3526 (56.1) | 1111 (56.7) | 1963 (50.4) | 2674 (61.5) | 2042 (60.4) | 2595 (53.4) | 382 (41.9) | 4255 (58.0) |
| North America | 1268 (20.2) | 545 (27.8) | 1033 (26.5) | 780 (17.9) | 683 (20.2) | 1130 (23.2) | 281 (30.8) | 1532 (20.9) |
| eGFR, mL/min/1.73 m2, mean (SD) b | 78.1 (20.1) | 69.3 (21.7) | 72.7 (20.8) | 78.9 (20.4) | 77.5 (21.1) | 74.9 (20.7) | 74.5 (21.3) | 76.2 (20.8) |
| eGFR, n (%) | ||||||||
| <60 mL/min/1.73 m2 | 1125 (17.9) | 682 (34.8) | 1056 (27.1) | 751 (17.3) | 682 (20.2) | 1125 (23.1) | 220 (24.1) | 1587 (21.6) |
| ≥60 to <90 mL/min/1.73 m2 | 3456 (55.0) | 934 (47.7) | 2026 (52.0) | 2364 (54.4) | 1809 (53.5) | 2581 (53.1) | 471 (51.7) | 3919 (53.4) |
| ≥90 mL/min/1.73 m2 | 1704 (27.1) | 344 (17.6) | 816 (20.9) | 1232 (28.3) | 891 (26.3) | 1157 (23.8) | 220 (24.1) | 1828 (24.9) |
| UACR, mg/g, mean (SD) c | 129.8 (459.5) | 204.4 (685.0) | 182.4 (594.6) | 116.1 (446.2) | 133.5 (493.0) | 157.2 (542.1) | 146.5 (505.7) | 147.5 (524.6) |
| Median UACR, mg/g c | 18.0 | 21.0 | 23.0 | 16.0 | 18.0 | 19.0 | 19.0 | 19.0 |
| UACR, n (%) | ||||||||
| <30 mg/g | 3705 (60.4) | 1078 (56.8) | 2076 (54.8) | 2707 (63.9) | 2013 (60.9) | 2770 (58.6) | 523 (59.6) | 4260 (59.6) |
| ≥30 to ≤300 mg/g | 1900 (31.0) | 592 (31.2) | 1267 (33.4) | 1225 (28.9) | 1015 (30.7) | 1477 (31.3) | 280 (31.9) | 2212 (30.9) |
| >300 mg/g | 527 (8.6) | 228 (12.0) | 448 (11.8) | 307 (7.2) | 278 (8.4) | 477 (10.1) | 74 (8.4) | 681 (9.5) |
| Duration of T2D, years, mean (SD) d | 12.5 (7.9) | 14.4 (9.5) | 16.2 (8.6) | 10.1 (6.9) | 11.7 (7.2) | 13.8 (8.9) | 13.3 (7.6) | 12.9 (8.4) |
| HbA1c, %, mean (SD) e | 8.2 (1.0) | 8.3 (1.0) | 8.4 (0.9) | 8.1 (1.0) | 8.2 (0.9) | 8.2 (1.0) | 8.2 (0.9) | 8.2 (1.0) |
| Patient history, n (%) | ||||||||
| Hypertension | 5740 (91.3) | 1783 (91.0) | 3595 (92.2) | 3928 (90.3) | 3053 (90.2) | 4470 (91.9) | 816 (89.6) | 6707 (91.4) |
| Dyslipidaemia | 4765 (75.8) | 1412 (72.0) | 3169 (81.3) | 3008 (69.2) | 2370 (70.1) | 3807 (78.3) | 770 (84.5) | 5407 (73.7) |
| Coronary artery disease | 4765 (75.8) | 1491 (76.1) | 2972 (76.2) | 3284 (75.5) | 2606 (77.0) | 3650 (75.1) | 722 (79.3) | 5534 (75.4) |
| Heart failure | 1413 (22.5) | 545 (27.8) | 904 (23.2) | 1054 (24.2) | 854 (25.2) | 1104 (22.7) | 122 (13.4) | 1836 (25.0) |
| Diabetic microvascular disease | 2296 (36.5) | 865 (44.1) | 1920 (49.3) | 1241 (28.5) | 1125 (33.3) | 2036 (41.9) | 359 (39.4) | 2802 (38.2) |
| Patients with use of diuretics, n (%) | 3170 (50.4) | 1068 (54.5) | 2253 (57.8) | 1985 (45.7) | 1678 (49.6) | 2560 (52.6) | 406 (44.6) | 3832 (52.2) |
| Number of GLAs, n (%) | ||||||||
| 0 | 1 (0.0) f | 105 (5.4) | 0 (0.0) | 106 (2.4) | 1 (0.0) f | 105 (2.2) | 0 (0.0) | 106 (1.4) |
| 1 | 1149 (18.3) | 1507 (76.9) | 1063 (27.3) | 1593 (36.6) | 420 (12.4) | 2236 (46.0) | 15 (1.6) | 2641 (36.0) |
| 2 | 3852 (61.3) | 304 (15.5) | 1993 (51.1) | 2163 (49.7) | 1985 (58.7) | 2171 (44.6) | 320 (35.1) | 3836 (52.3) |
| ≥3 | 1284 (20.4) | 44 (2.2) | 842 (21.6) | 486 (11.2) | 977 (28.9) | 351 (7.2) | 576 (63.2) | 752 (10.3) |
| Patients with use of | ||||||||
| Metformin, n (%) | 6286 (100.0) | 0 (0.0) | 2574 (66.0) | 3712 (85.4) | 2752 (81.3) | 3534 (72.7) | 766 (84.1) | 5520 (75.3) |
| Insulin, n (%) | 2574 (40.9) | 1324 (67.6) | 3898 (100.0) | 0 (0.0) | 639 (18.9) | 3259 (67.0) | 287 (31.5) | 3611 (49.2) |
| SUs, n (%) | 2752 (43.8) | 631 (32.2) | 639 (16.4) | 2744 (63.1) | 3383 (100.0) | 0 (0.0) | 443 (48.6) | 2940 (40.1) |
| DPP‐4 inhibitors, n (%) | 766 (12.2) | 145 (7.4) | 287 (7.4) | 624 (14.4) | 443 (13.1) | 468 (9.6) | 911 (100.0) | 0 (0.0) |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; DPP‐4, dipeptidyl peptidase‐4; GLA, glucose‐lowering agent; HbA1c, glycated haemoglobin; SD, standard deviation; SU, sulphonylurea; T2D, type 2 diabetes; UACR, urine albumin to creatinine ratio.
N = 4513 and 1345 for with and without metformin, respectively; N = 2737 and 3121 for with and without insulin, respectively; N = 2420 and 3438 for with and without SUs, respectively; N = 656 and 5202 for with and without DPP‐4 inhibitors, respectively.
N = 6285 and 1960 for with and without metformin, respectively; N = 3898 and 4347 for with and without insulin, respectively; N = 3382 and 4863 for with and without SUs, respectively; N = 911 and 7334 for with and without DPP‐4 inhibitors, respectively.
N = 6132 and 1898 for with and without metformin, respectively; N = 3791 and 4239 for with and without insulin, respectively; N = 3306 and 4724 for with and without SUs, respectively; N = 877 and 7153 for with and without DPP‐4 inhibitors, respectively.
N = 6283 and 1955 for with and without metformin, respectively; N = 3895 and 4343 for with and without insulin, respectively; N = 3381 and 4857 for with and without SUs, respectively; N = 908 and 7330 for with and without DPP‐4 inhibitors, respectively.
N = 6259 and 1947 for with and without metformin, respectively; N = 3876 and 4330 for with and without insulin, respectively; N = 3369 and 4837 for with and without SUs, respectively; N = 906 and 7300 for with and without DPP‐4 inhibitors, respectively.
Number of GLAs was summarized at screening, whereas the GLA subgroup allocation was determined at baseline. One patient started metformin therapy and one patient started SU therapy between screening and baseline.
3.2. Cardiorenal outcomes
The effects of ertugliflozin on selected prespecified CV and kidney outcomes in patients with T2DM and prevalent ASCVD were not modified by baseline use of any of the glucose‐lowering agents (Figure 1). There was no significant difference in the effect of ertugliflozin on the primary MACE outcome in patients with versus without metformin (with: HR 0.92 [95% CI 0.790, 1.073]; without: 1.13 [95% CI 0.867, 1.480]), insulin (with: 0.91 [95% CI 0.765, 1.092]; without: 1.06 [95% CI 0.867, 1.293]), SU (with: 1.11 [95% CI 0.890, 1.388; without: 0.90 [95% CI 0.761, 1.060]) or DPP‐4 inhibitor use (with: 0.77 [95% CI 0.502, 1.173; without: 1.00 [95% CI 0.867, 1.147]) (all interaction P > 0.05).
FIGURE 1.
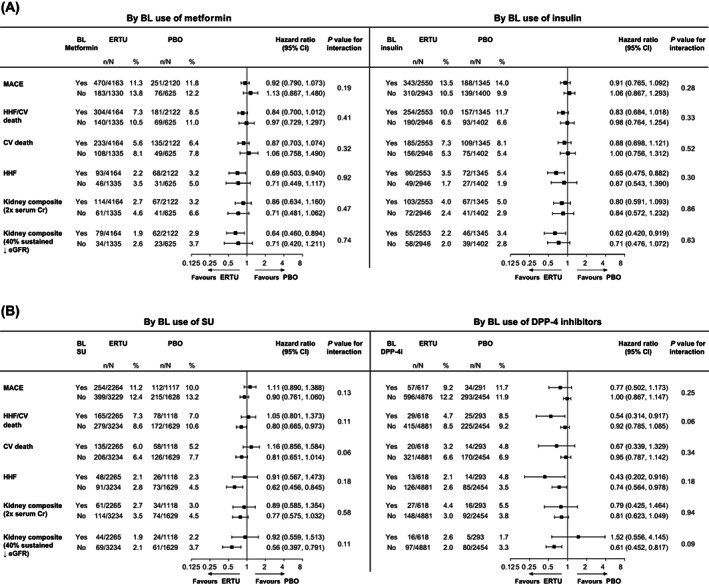
Cardiovascular (CV) and kidney outcomes with ertugliflozin versus placebo by baseline (A) metformin and insulin, (B) sulphonylurea (SU) and dipeptidyl peptidase‐4 (DPP‐4) inhibitor use. The analysis of major adverse CV events (MACE) was performed with data from all the patients who had undergone randomization and received at least one dose of ertugliflozin (n = 5493) or placebo (n = 2745). For patients who permanently discontinued the trial regimen prematurely, only MACE that occurred up to 365 days after the confirmed last dose were included in the primary analysis. The analyses of the other outcomes were performed on an intention‐to‐treat basis with data from all the patients who had undergone randomization to receive ertugliflozin (n = 5499) or placebo (n = 2747), and all time on‐study for each patient. The interaction P value is shown for the two‐level treatment group (all ertugliflozin vs. placebo). BL, baseline; CI, confidence interval; Cr, creatinine; eGFR, estimated glomerular filtration rate; ERTU, ertugliflozin; HHF, hospitalization for heart failure; PBO, placebo
Findings for secondary outcomes were similar for each baseline glucose‐lowering agent class evaluated. There was no significant difference in the effect of ertugliflozin on HHF in patients treated with versus without metformin (with: HR 0.69 [95% CI 0.503, 0.940]; without: 0.71 [95% CI 0.449, 1.117]), insulin (with: 0.65 [95% CI 0.475, 0.882]; without: 0.87 [95% CI 0.543, 1.390]), SUs (with: 0.91 [95% CI 0.567, 1.473]; without: 0.62 [95% CI 0.456, 0.845]) or DPP‐4 inhibitors (with: 0.43 [95% CI 0.202, 0.916]; without: 0.74 [95% CI 0.564, 0.978]) (all interaction P > 0.05). Also, the effects of ertugliflozin on the kidney composite outcome of death from kidney causes, kidney replacement therapy, or doubling of the serum creatinine level were similar in patients with versus without metformin (with: HR 0.86 [95% CI 0.634, 1.160]; without: 0.71 [95% CI 0.481, 1.062]), insulin (with: 0.80 [95% CI 0.591, 1.093]; without: 0.84 [95% CI 0.572, 1.232]), SU (with: 0.89 [95% CI 0.585, 1.354]; without: 0.77 [95% CI 0.575, 1.032]) or DPP‐4 inhibitor use (with: 0.79 [95% CI 0.425, 1.464]; without: 0.81 [95% CI 0.623, 1.049]) (all interaction P > 0.05), as was the effect on the kidney composite outcome of a sustained decrease of 40% or more in eGFR to less than 60 mL/min/1.73 m2, new end‐stage kidney disease, or death from kidney causes (metformin, with: HR 0.64 [95% CI 0.460, 0.894], without: 0.71 [95% CI 0.420, 1.211]; insulin, with: 0.62 [95% CI 0.420, 0.919], without: 0.71 [95% CI 0.476, 1.072]; SUs, with: 0.92 [95% CI 0.559, 1.513], without: 0.56 [95% CI 0.397, 0.791]; DPP‐4 inhibitors, with: 1.52 [95% CI 0.556, 4.145], without: 0.61 [95% CI 0.452, 0.817]) (all interaction P > 0.05).
There was no significant difference in the effect of ertugliflozin versus placebo on cardiorenal outcomes by the number of glucose‐lowering agents used at baseline (all interaction P > 0.05; data not shown).
3.3. Metabolic outcomes
Reductions in HbA1c, body weight and SBP were greater with ertugliflozin versus placebo over the study duration and the magnitude of ertugliflozin's efficacy were not notably different across these clinical assessments based on baseline treatment with metformin, insulin, SUs or DPP‐4 inhibitors (Figures S1‐S3).
3.4. Renal function
The overall pattern of eGFR changes with ertugliflozin versus placebo over the course of the study was consistent across all drug classes of baseline glucose‐lowering agents. An initial decline in eGFR was observed with ertugliflozin versus placebo, with subsequent stabilization of eGFR levels, and final eGFR levels at Year 5 were higher with ertugliflozin versus placebo (Figure 2). The pattern of change in UACR levels over the course of the study was consistent across all classes of baseline glucose‐lowering agent. Ertugliflozin was associated with a reduction in UACR versus placebo across the different glucose‐lowering agent classes, which persisted for the duration of the study (Figure S4).
FIGURE 2.
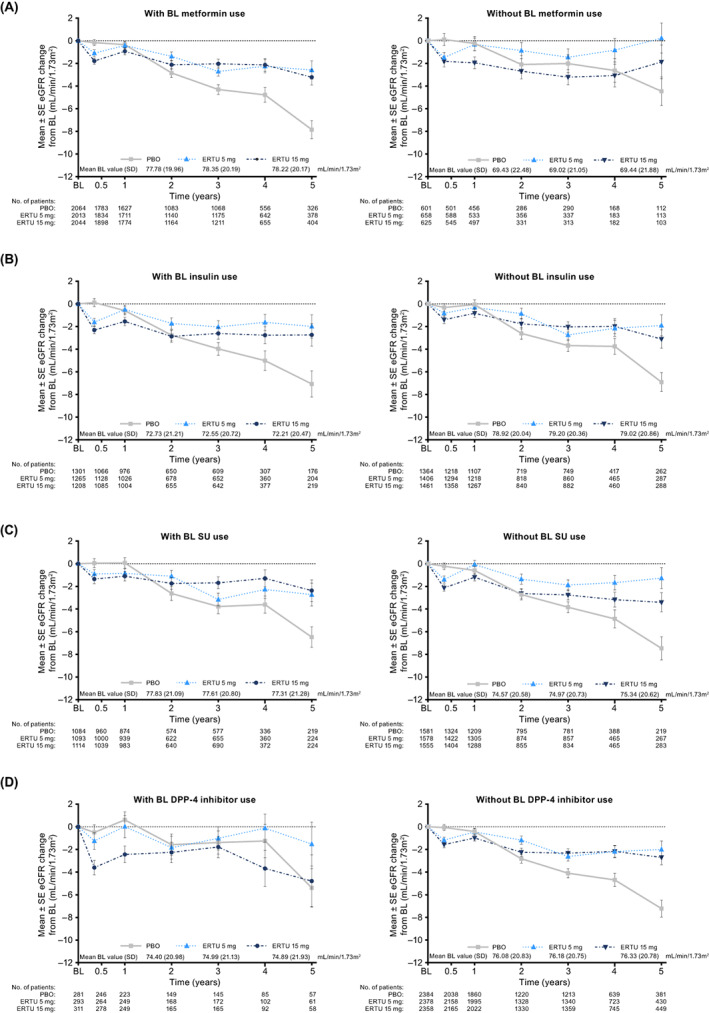
Changes in estimated glomerular filtration rate (eGFR) over time with ertugliflozin versus placebo by baseline (A) metformin, (B) insulin, (C) sulphonylurea (SU) and (D) dipeptidyl peptidase‐4 (DPP‐4) inhibitor use. Mean (standard deviation [SD]) baseline eGFR levels by treatment are shown in the key for each baseline glucose‐lowering class. N is the number of patients without missing data at each time point. BL, baseline; ERTU, ertugliflozin; PBO, placebo; SE, standard error
4. DISCUSSION
These post hoc analyses demonstrated that the effects of ertugliflozin on prespecified CV and kidney outcomes in patients with T2DM and prevalent ASCVD were consistent across participants stratified by baseline glucose‐lowering agent use. No significant difference was observed in the effect of ertugliflozin on the primary MACE outcome across baseline glucose‐lowering agent use. Notably, the reductions in risk for HHF and the kidney composite outcome of a sustained decrease of 40% or more in eGFR to less than 60 mL/min/1.73 m2, new end‐stage kidney disease or death from kidney causes observed with ertugliflozin versus placebo in the overall VERTIS CV population 10 , 11 , 13 were observed, regardless of baseline glucose‐lowering agent. These data support the concept that the beneficial effect of ertugliflozin is consistent irrespective of therapeutic agent(s) used as baseline medication.
The results observed in this study align with analyses of CVOTs of other SGLT2 inhibitors. In a prespecified analysis of the EMPA‐REG OUTCOME trial, the addition of empagliflozin to existing glucose‐lowering regimens of patients with T2DM and CV disease reduced their risks of adverse CV outcomes and mortality irrespective of baseline use of metformin, SUs or insulin. 14 However, in that study, there was a suggestion that empagliflozin may have a greater benefit of attenuating chronic kidney disease progression in patients not taking metformin at baseline. In a post hoc analysis of DECLARE TIMI 58, the effects of dapagliflozin on cardiorenal outcomes in patients with T2DM and ASCVD or multiple risk factors for CV disease were reported to be consistent regardless of baseline use of metformin, SUs, DPP‐4 inhibitors or insulin. 15
In keeping with previously published VERTIS studies, ertugliflozin led to greater reductions in HbA1c, body weight and SBP compared with placebo. 16 , 17 , 18 , 19 , 20 , 21 , 22 These reductions were irrespective of baseline glucose‐lowering agent, supporting the glycaemic, metabolic and haemodynamic benefits of adding ertugliflozin to any existing glucose‐lowering regimens. Changes in HbA1c, body weight and SBP with ertugliflozin versus placebo in each baseline glucose‐lowering agent class were similar to those observed in the overall VERTIS CV population. 10
The pattern of initial dip in eGFR with ertugliflozin treatment in all baseline glucose‐lowering agent drug classes, followed by stabilization, is consistent with the results from the overall VERTIS CV population. 11 The change in UACR in all baseline glucose‐lowering agent classes observed in these analyses is also consistent with the results from the overall VERTIS CV population. 11 The slowing of eGFR decline and lowering of UACR has also been reported with other SGLT2 inhibitors, 5 , 6 , 23 , 24 with an analysis of the DECLARE TIMI study by baseline glucose‐lowering agent showing similar results. 15 Together with the reduction in risk for the composite kidney outcome, these data emphasize the effectiveness of SGLT2 inhibitors in reducing the risk for progression of diabetic kidney disease, regardless of choice of initial glucose‐lowering therapy.
International guidelines for patients with T2DM have been updated over recent years not only to reflect the greater choice of therapies but also to recognize the results from the recent CVOTs of SGLT2 inhibitors and glucagon‐like peptide‐1 (GLP‐1) receptor agonists. Some differences in the role of metformin as first‐line therapy exist between the recommendations from the American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD), Kidney Disease Improving Global Outcomes (KDIGO) and the 2019 guidelines from the European Society of Cardiology (ESC). 25 , 26 , 27 While current clinical practice recommendations from the ADA propose a patient‐centric approach to guide the choice of pharmacological agents, with considerations such as CV and kidney comorbidities, hypoglycaemia risk, impact on weight, cost, risk of side effects and patient preferences, metformin is still the preferred initial pharmacological agent for the treatment of patients with T2DM. 25 However, the results from the present analyses, together with data from the analyses of the EMPA‐REG OUTCOME 14 and DECLARE TIMI 5815 trials by baseline glucose‐lowering agent, suggest no difference in outcomes with or without metformin use and therefore add support to the use of SGLT2 inhibitors earlier in the treatment paradigm. On the basis of CVOTs of SGLT2 inhibitors 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 and GLP‐1 receptor agonists, 28 , 29 , 30 , 31 , 32 the 2019 ESC guidelines on diabetes and CV diseases recommended a shift in focus from a glucose‐centric to a cardio‐centric approach for patients with ASCVD or at high/very high CV risk with the initiation of a GLP‐1 receptor agonist or a SGLT2 inhibitor in drug‐naïve patients or the addition of a GLP‐1 receptor agonist or a SGLT2 inhibitor in patients already on metformin. 26 From a kidney perspective, the 2020 KDIGO clinical practice guidelines recommend metformin and an SGLT2 inhibitor as first‐line treatment in patients with T2D and chronic kidney disease. 27 The body of scientific evidence, along with updated guidelines, will help ensure that patients with T2DM, CV disease, heart failure or chronic kidney disease are treated with an SGLT2 inhibitor or GLP‐1 receptor agonist when appropriate, independent of background therapy, to optimize cardiorenal health. 33
There are some potential limitations to the present report. The VERTIS CV population was predominantly White (88%) and male (70%) and this should be considered when interpreting the findings presented. There were some differences in baseline characteristics between subgroups of patients according to baseline use of glucose‐lowering medications (eg, duration of diabetes and eGFR at enrolment), which might obscure an effect of background medication on observed ertugliflozin effects. The potential influence of baseline characteristics on ertugliflozin effects has been further explored in the case of metformin in a separate analysis using propensity adjustment by inverse probability of treatment weighting. 34 Also, the proportion of patients treated with GLP‐1 at baseline was too small (3.4% in the overall VERTIS CV population) 10 for meaningful subgroup analysis. Additionally, the present analyses do not consider any changes in usage or dose of glucose‐lowering medication during the study, and do not include any adjustment for monotherapy versus combination therapy. Despite these limitations, our findings confirm and extend those from previous SGLT2 inhibitor outcome trials by showing consistent glycaemic, metabolic, haemodynamic and cardiorenal effects of ertugliflozin in participants receiving different regimens for glycaemic control at baseline.
In conclusion, in VERTIS CV, the effects of ertugliflozin on selected CV and kidney outcomes in patients with T2DM and prevalent ASCVD were not modified by baseline use of glucose‐lowering agent. The magnitude of the reductions in HbA1c, body weight, SBP, eGFR and UACR with ertugliflozin were not notably different across baseline glucose‐lowering agent class.
CONFLICTS OF INTEREST
S.D.‐J. has led clinical trials for AstraZeneca, Boehringer Ingelheim and Novo Nordisk, has received consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, Janssen, Merck & Co. and Sanofi, and has equity interests in Jana Care and Aerami Therapeutics. C.P.C. has received research grants from Amgen, Better Therapeutics, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Janssen, Merck and Pfizer, fees from Aegerion/Amryt, Alnylam, Amarin, Amgen, Applied Therapeutics, Ascendia, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, Janssen, Lexicon, Merck, Pfizer, Rhoshan and Sanofi, and has served on data and safety monitoring boards for the Veteran's Administration, Applied Therapeutics and Novo Nordisk. D.Z.I.C. has received consulting fees and/or speaking honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Merck & Co., Mitsubishi‐Tanabe, Novo Nordisk, Prometic and Sanofi, and has received operating funds from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck & Co., Novo Nordisk and Sanofi. F.C. has received research grants from the Swedish Research Council, Swedish Heart & Lung Foundation and King Gustav V and Queen Victoria Foundation, and has received consulting fees from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Merck Sharp & Dohme, Mundipharma, Novo Nordisk and Pfizer. R.E.P. has received grants (directed to his institution) from Hanmi Pharmaceutical, Janssen, Metavention, Novo Nordisk, Poxel and Sanofi, consulting fees (directed to his institution) from AstraZeneca, Corcept Therapeutics, Glytec, Hanmi Pharmaceutical, Janssen, Merck & Co., Mundipharma, Novo Nordisk, Pfizer, Sanofi, Scohia Pharma and Sun Pharma, and support for attending meetings/travel (directed to his institution or to the travel provider) from AstraZeneca, Glytec, Merck & Co., Mundipharma, Novo Nordisk and Pfizer. J.L., A.P. and I.G. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, who own stock in Merck & Co., Inc., Kenilworth, New Jersey. R.F. and J.P.M. are employees and shareholders of Pfizer Inc.
AUTHOR CONTRIBUTIONS
All authors critically reviewed the draft manuscript and approved the final version of the manuscript for publication. Jie Liu, Annpey Pong, Ira Gantz, Robert Frederich and James P. Mancuso were involved in the conception/design of the study. Samuel Dagogo‐Jack, Christopher P. Cannon, David Z. I. Cherney, Francesco Cosentino, and Richard E. Pratley were involved in the conduct of the study. All authors were involved in data analysis and interpretation of the data. This research was sponsored by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey in collaboration with Pfizer Inc., New York, New York. Editorial support was provided by Marion James, PhD, CMPP, of Engage Scientific Solutions (Horsham, UK) and was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, in collaboration with Pfizer Inc., New York, New York
Supporting information
Appendix S1 Supporting information
ACKNOWLEDGMENTS
The authors thank the investigators, staff and participants of the VERTIS CV study (Protocol B1521021) and Bernard Charbonnel, Université de Nantes, France, for presenting these data in part at the 81st Scientific Sessions of the ADA and the 57th Annual Meeting of the EASD. The authors also thank Ingrid Adamsons of Merck & Co., Inc., Kenilworth, New Jersey, Philip Jones of Pfizer Inc., Sandwich, United Kingdom, and Ahmed Kolkailah of University of Texas Southwestern Medical Center, Texas for their critical review of this manuscript.
Dagogo‐Jack S, Cannon CP, Cherney DZI, et al. Cardiorenal outcomes with ertugliflozin assessed according to baseline glucose‐lowering agent: An analysis from VERTIS CV . Diabetes Obes Metab. 2022;24(7):1245-1254. doi: 10.1111/dom.14691
Funding information Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, in collaboration with Pfizer Inc., New York, NY, USA
DATA AVAILABILITY STATEMENT
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA's data sharing policy, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
REFERENCES
- 1. American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43:S98‐S110. [DOI] [PubMed] [Google Scholar]
- 2. Filippas‐Ntekouan S, Filippatos TD, Elisaf MS. SGLT2 inhibitors: are they safe? Postgrad Med. 2018;130:72‐82. [DOI] [PubMed] [Google Scholar]
- 3. Hsia DS, Grove O, Cefalu WT. An update on sodium‐glucose co‐transporter‐2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24:73‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 5. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644‐657. [DOI] [PubMed] [Google Scholar]
- 6. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347‐357. [DOI] [PubMed] [Google Scholar]
- 7. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413‐1424. [DOI] [PubMed] [Google Scholar]
- 8. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295‐2306. [DOI] [PubMed] [Google Scholar]
- 9. Heerspink HJL, Stefánsson BV, Correa‐Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436‐1446. [DOI] [PubMed] [Google Scholar]
- 10. Cannon CP, Pratley R, Dagogo‐Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425‐1435. [DOI] [PubMed] [Google Scholar]
- 11. Cherney DZI, Charbonnel B, Cosentino F, et al. Effects of ertugliflozin on kidney composite outcomes, renal function and albuminuria in patients with type 2 diabetes mellitus: an analysis from the randomised VERTIS CV trial. Diabetologia. 2021;64:1256‐1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cannon CP, McGuire DK, Pratley R, et al. Design and baseline characteristics of the eValuation of ERTugliflozin effIcacy and safety CardioVascular outcomes trial (VERTIS‐CV). Am Heart J. 2018;206:11‐23. [DOI] [PubMed] [Google Scholar]
- 13. Cosentino F, Cannon CP, Cherney DZI, et al. Efficacy of ertugliflozin on heart failure‐related events in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease: results of the VERTIS CV trial. Circulation. 2020;142:2205‐2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Inzucchi SE, Fitchett D, Jurisic‐Erzen D, et al. Are the cardiovascular and kidney benefits of empagliflozin influenced by baseline glucose‐lowering therapy? Diabetes Obes Metab. 2020;22:631‐639. [DOI] [PubMed] [Google Scholar]
- 15. Cahn A, Wiviott SD, Mosenzon O, et al. Cardiorenal outcomes with dapagliflozin by baseline glucose‐lowering agents: post hoc analyses from DECLARE‐TIMI 58. Diabetes Obes Metab. 2021;23:29‐38. [DOI] [PubMed] [Google Scholar]
- 16. Terra SG, Focht K, Davies M, et al. Phase III, efficacy and safety study of ertugliflozin monotherapy in people with type 2 diabetes mellitus inadequately controlled with diet and exercise alone. Diabetes Obes Metab. 2017;19:721‐728. [DOI] [PubMed] [Google Scholar]
- 17. Dagogo‐Jack S, Liu J, Eldor R, et al. Efficacy and safety of the addition of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sitagliptin: the VERTIS SITA2 placebo‐controlled randomized study. Diabetes Obes Metab. 2018;20:530‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grunberger G, Camp S, Johnson J, et al. Ertugliflozin in patients with stage 3 chronic kidney disease and type 2 diabetes mellitus: the VERTIS RENAL randomized study. Diabetes Ther. 2018;9:49‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hollander P, Liu J, Hill J, et al. Ertugliflozin compared with glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin: the VERTIS SU randomized study. Diabetes Ther. 2018;9:193‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller S, Krumins T, Zhou H, et al. Ertugliflozin and sitagliptin co‐initiation in patients with type 2 diabetes: the VERTIS SITA randomized study. Diabetes Ther. 2018;9:253‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pratley RE, Eldor R, Raji A, et al. Ertugliflozin plus sitagliptin versus either individual agent over 52 weeks in patients with type 2 diabetes mellitus inadequately controlled with metformin: the VERTIS FACTORIAL randomized trial. Diabetes Obes Metab. 2018;20:1111‐1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosenstock J, Frias J, Páll D, et al. Effect of ertugliflozin on glucose control, body weight, blood pressure and bone density in type 2 diabetes mellitus inadequately controlled on metformin monotherapy (VERTIS MET). Diabetes Obes Metab. 2018;20:520‐529. [DOI] [PubMed] [Google Scholar]
- 23. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323‐334. [DOI] [PubMed] [Google Scholar]
- 24. Cherney DZI, Zinman B, Inzucchi SE, et al. Effects of empagliflozin on the urinary albumin‐to‐creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA‐REG OUTCOME randomised, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2017;5:610‐621. [DOI] [PubMed] [Google Scholar]
- 25. American Diabetes A . 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2021. Diabetes Care. 2021;44:S111‐S124. [DOI] [PubMed] [Google Scholar]
- 26. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255‐323. [DOI] [PubMed] [Google Scholar]
- 27. Kidney Disease: Improving Global Outcomes Diabetes Work G . KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98:S1‐S115. [DOI] [PubMed] [Google Scholar]
- 28. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 30. Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double‐blind, randomised placebo‐controlled trial. Lancet. 2018;392:1519‐1529. [DOI] [PubMed] [Google Scholar]
- 31. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double‐blind, randomised placebo‐controlled trial. Lancet. 2019;394:121‐130. [DOI] [PubMed] [Google Scholar]
- 32. Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841‐851. [DOI] [PubMed] [Google Scholar]
- 33. Marx N, Davies MJ, Grant PJ, et al. Guideline recommendations and the positioning of newer drugs in type 2 diabetes care. Lancet Diabetes Endocrinol. 2021;9:46‐52. [DOI] [PubMed] [Google Scholar]
- 34. Cosentino F, Cannon CP, Cherney DZI, et al. Cardiorenal outcomes with ertugliflozin by baseline metformin use: post‐hoc analyses of the VERTIS CV trial. Eur Heart J. 2021;42:ehab724.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting information
Data Availability Statement
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA's data sharing policy, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.


