FIGURE 1.
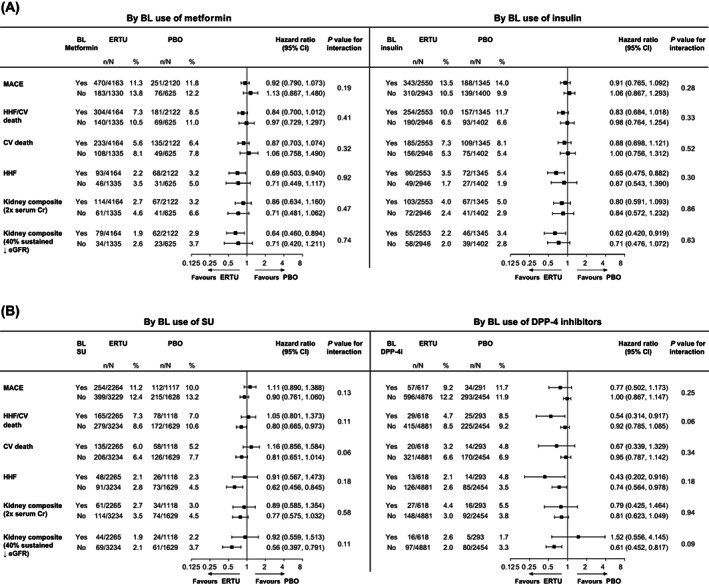
Cardiovascular (CV) and kidney outcomes with ertugliflozin versus placebo by baseline (A) metformin and insulin, (B) sulphonylurea (SU) and dipeptidyl peptidase‐4 (DPP‐4) inhibitor use. The analysis of major adverse CV events (MACE) was performed with data from all the patients who had undergone randomization and received at least one dose of ertugliflozin (n = 5493) or placebo (n = 2745). For patients who permanently discontinued the trial regimen prematurely, only MACE that occurred up to 365 days after the confirmed last dose were included in the primary analysis. The analyses of the other outcomes were performed on an intention‐to‐treat basis with data from all the patients who had undergone randomization to receive ertugliflozin (n = 5499) or placebo (n = 2747), and all time on‐study for each patient. The interaction P value is shown for the two‐level treatment group (all ertugliflozin vs. placebo). BL, baseline; CI, confidence interval; Cr, creatinine; eGFR, estimated glomerular filtration rate; ERTU, ertugliflozin; HHF, hospitalization for heart failure; PBO, placebo
