Abstract
Aim
To examine the cross‐sectional associations between single nutrient intakes and posteriori nutrient‐based dietary patterns and periodontal disease risk in a subset of the UK Biobank cohort.
Materials and Methods
Dietary data were collected by 24‐h dietary recall on up to five separate occasions over 16 months. A touchscreen questionnaire was used to collect oral health information. Participants were considered at high risk of periodontal disease if they reported having painful gums and/or bleeding gums and/or loose teeth. Principal component analysis identified four nutrient‐based dietary patterns from 20 nutrients. Logistic regression was used to estimate the odds ratio of periodontal disease risk for single nutrients and nutrient‐based dietary patterns.
Results
A total of 9476 participants (mean age 56.2 years [SD 8.0]) were included in the analysis. Higher intakes of vitamin B6, B12, C, and E, folate, iron, potassium, magnesium, polyunsaturated fatty acids, and total sugar were associated with a lower risk of periodontal disease. Higher intake of saturated fat was associated with an increased risk. A dietary pattern characterized by high micronutrients and fibre intake was associated with low risk of periodontal disease.
Conclusion
Within this sample of middle‐aged and older adults, a “high micronutrient and fibre” dietary pattern was associated with reduced risk of periodontal disease.
Keywords: dietary pattern, epidemiology, nutrient intake, periodontal disease, periodontitis
Clinical Relevance.
Scientific rationale for study: Evidence supporting the relationship between dietary intake and periodontal disease has predominately focused on single nutrients; however, nutrients are not consumed in isolation. There is a lack of studies investigating the relationship between dietary patterns created using nutrient intakes and the risk of periodontal disease.
Principal findings: A dietary pattern characterized by high micronutrients and fibre intake may reduce the risk of periodontal disease.
Practical implications: Future studies investigating the relationship between nutrient‐based dietary patterns and periodontal disease are warranted to enhance the understanding of the key biological processes and help formulate dietary recommendations to reduce periodontal disease risk.
1. INTRODUCTION
Periodontal disease, including gingivitis and periodontitis, is an inflammatory condition that affects the tissues that surround and support the tooth (Kinane et al., 2017). It is a major cause of tooth loss, which consequently can lead to poor nutritional status, low self‐esteem, and reduced quality of life (Tonetti et al., 2017). A growing body of evidence also suggests that periodontal disease is associated with several conditions, including diabetes, cardiovascular diseases, cancer, rheumatoid arthritis, pre‐eclampsia, dementia, and Alzheimer's disease (Chapple et al., 2013; Tonetti et al., 2013; Bourgeois et al., 2019; Nadim et al., 2020; Bora et al., 2021; Gare et al., 2021; Maitre et al., 2021). Periodontal diseases are among the most prevalent chronic infections in humans. The global age‐standardized prevalence of severe periodontitis alone was 9.8% in 2017, while the number of prevalent cases was 796 million worldwide (Bernabe et al., 2020).
Dietary intake has been considered a modifiable risk factor for periodontal disease (Hujoel & Lingström, 2017). For instance, low intakes of dietary fibre, omega‐3 fatty acids, vitamin A and its precursor β‐carotene, vitamin B12, vitamin C, and calcium, as well as high intakes of saturated fat and fermented carbohydrates, have all been associated with an increased risk of periodontitis (O'Connor et al., 2020; Martinon et al., 2021). To date, the evidence supporting the relationship between dietary intake and periodontal disease has predominately focused on single nutrients. However, nutrients are not consumed in isolation, and therefore analysing the effect of single nutrients fails to take into account the complex interactions that occur among them. It has been suggested that analysing the diet as a whole may be more predictive of disease risk (Hu, 2002).
A dietary pattern analysis is a complementary approach to single nutrient analysis and provides a more comprehensive overview of the diet (Hu, 2002). The method involves the grouping of nutrients, foods, or food groups that are commonly consumed together. A number of studies have used dietary pattern analysis to examine the relationship between diet and periodontal disease (Al‐Zahrani et al., 2005; Bawadi et al., 2011; Jauhiainen et al., 2016, 2020; Salazar et al., 2018; Wright et al., 2020; Alhassani et al., 2021; Li et al., 2021). Most of these studies have used a “priori” approach, which evaluates dietary intake using pre‐defined scoring classifications based on dietary guidelines or knowledge of diet and disease relationships. For instance, consumption of a healthy diet measured by the Baltic Sea Diet Score (Jauhiainen et al., 2016, 2020), the Recommended Finnish Diet Score (Jauhiainen et al., 2016, 2020), the Healthy Eating Index (Al‐Zahrani et al., 2005; Bawadi et al., 2011), and the Alternative Healthy Eating Index (Salazar et al., 2018) have all been associated with a reduced risk of periodontal disease, whereas consuming a pro‐inflammatory diet measured by the energy‐adjusted Dietary Inflammatory Index has been associated with periodontitis in US adults (Li et al., 2021).
Another approach to dietary pattern analysis is the “posteriori” approach, which is a data‐driven method that involves the grouping of highly correlated nutrients, foods, or food groups using a data reduction technique. Although the dietary patterns identified using this approach are specific to the population from which they are derived, it is common for both “prudent‐type” and “Western‐type” dietary patterns to be identified. Only a few studies have used this dietary pattern analysis approach when investigating the relationship between diet and periodontal disease. The results from these studies, however, are conflicting, as one study found a significant association between a “prudent‐type” dietary pattern and a decreased risk of periodontal disease (Wright et al., 2020), while another study found no association (Alhassani et al., 2021).
The dietary patterns identified in the above studies were created using food groups, which can be easily translated into dietary recommendations. To the best of our knowledge, however, no study has investigated the association between dietary patterns created using nutrient intakes and the risk of periodontal disease. Using nutrient intakes to identify dietary patterns may enhance the understanding of the key biological processes and allow easier comparisons across populations.
2. AIM
We aimed to investigate the cross‐sectional associations between single nutrient intakes and posteriori nutrient‐based dietary patterns with periodontal disease risk in the UK Biobank Cohort.
3. METHODS
3.1. Participants and study design
The UK Biobank is a prospective population‐based cohort study. About half a million UK adults (5.5% response rate) aged between 40 and 69 years were recruited between 2006 and 2010. These participants attended an assessment centre where a touchscreen questionnaire and a brief computer‐assisted interview were completed and a range of physical assessments were made. Participants also provided biological specimens. Further information regarding the study design and methodology is provided in the study protocol (UK Biobank, 2007). All participants provided informed consent.
An application was submitted to UK Biobank requesting data for all participants who had dietary intake data captured by the 24‐h recalls and/or food frequency questionnaire and/or oral health data captured by the touchscreen questionnaire (n = 135,428). Thirty‐eight participants were excluded from the analysis because they had withdrawn from the UK Biobank study at the time of analysis. Participants were also excluded if they had missing oral health and nutrient intake data (calculated from the 24‐h dietary recall assessment only) (n = 77,921); preferred not to answer the oral health questions (n = 497); did not complete the first 24‐h dietary recall questionnaire at the assessment centre and at least one of the online 24‐h dietary recalls (n = 47,311); had reported an implausible energy intake (<500 or >3500 kcal/day for women and <800 or >4000 kcal/day for men) (n = 100); and had missing covariates (age, sex, ethnicity, Townsend Deprivation Index [TDI], body mass index [BMI], smoking status, and alcohol drinking status) (n = 85). A total of 9476 participants were available for analysis. The current analysis used only data collected at baseline. A flowchart of study participants is presented in Figure 1.
FIGURE 1.
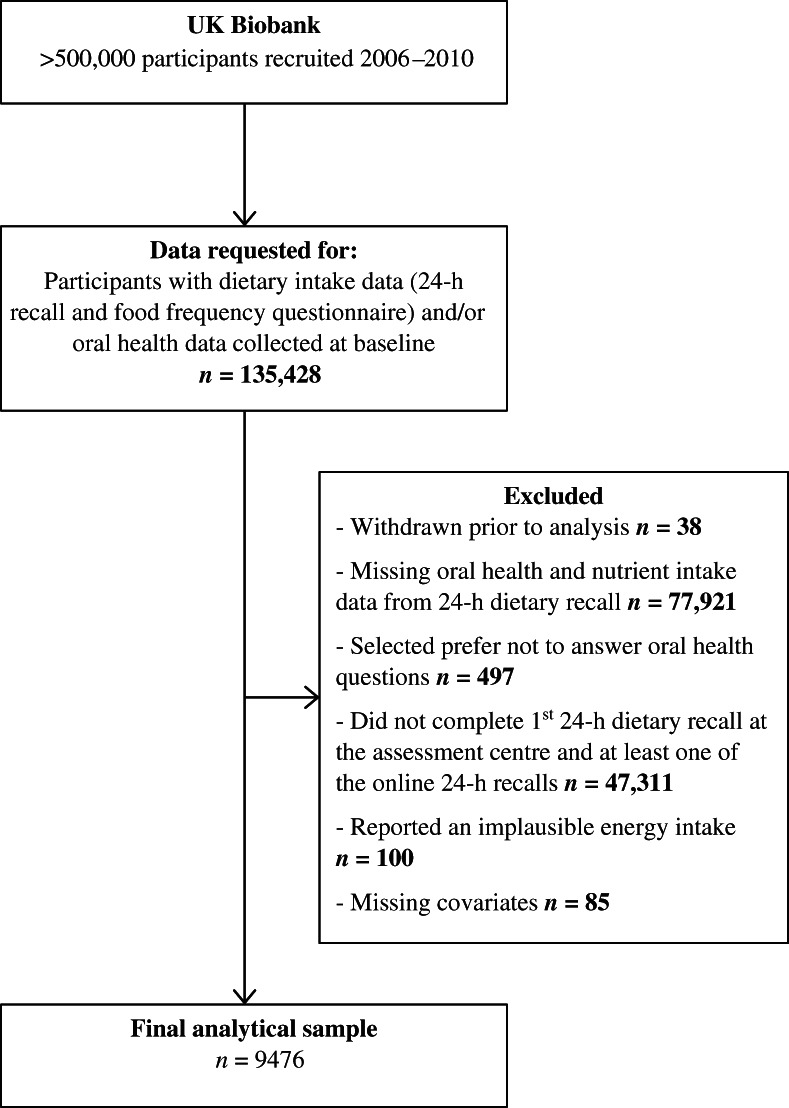
Flowchart of study participants
3.2. Periodontal disease risk
Self‐reported periodontal disease information was collected by the touchscreen questionnaire. A clinical dental examination was not included. Participants were asked to indicate if they had painful gums, bleeding gums, or loose teeth. Participants could select more than one option or could select “none of the above”. There was also an option “prefer not to answer”. Self‐reported painful gums, bleeding, gums, and loose teeth have previously been validated as surrogate makers of periodontal disease (Eke et al., 2013; Abbood et al., 2016). Participants were considered at increased risk of periodontal disease if they reported having one or more of the symptoms. Those who did not report any of the symptoms were considered at low risk of periodontal disease.
3.3. Dietary intake
Towards the end of recruitment, the Oxford WebQ, a web‐based 24‐h dietary assessment tool, was added to the baseline assessment protocol. The web‐based dietary assessment tool records the consumption of up to 206 widely consumed foods and 32 types of beverages in the previous 24 h. Standard categories were used for portion sizes (e.g., two slices of bread), and for foods without a natural size (e.g., rice), “serving” sizes with a description were provided (Liu et al., 2011).
Participants recruited between April 2009 and June 2010 were asked to complete the 24‐h dietary assessment at the assessment centre. In addition, participants who provided an email address at the assessment centre were sent, on four separate occasions between February 2011 and June 2012, an invite to complete an identical online version of the 24‐h recall assessment using their own computer.
The UK Biobank provided the data for energy intake and 20 nutrients, all of which have been included in the current analysis. Nutritional supplement intake was not used when estimating daily nutrient intakes. Mean daily intakes of nutrients were calculated by using standard portion sizes for each food or beverage consumed and by multiplying the amount consumed by the nutrient composition. Participants who completed two or more 24‐h dietary assessments had their values averaged. For the current analysis, the mean daily nutrient intake values were expressed as mean intakes per 1000 kcal of total energy intake.
3.4. Covariates
Anthropometric measures including weight (kilogram) and height (centimetre) were taken at the assessment centre and were used to calculate the BMI (kilogram per square metre). The touchscreen questionnaire collected socio‐demographic information including age, sex, ethnicity, and TDI, which is a proxy measure for socio‐economic status. TDI was based on the preceding national census output area. Participants were assigned a score corresponding to their area of residence, with a greater score indicating a greater degree of deprivation. Smoking status (current, previous, or never) and alcohol drinking status (current, previous, or never) were also collected by the touchscreen questionnaire.
3.5. Statistical methods
Data analysis was performed using SPSS for Windows version 27.0 (IBM Corp., Armonk, NY). Sample characteristics are presented as frequencies and percentages for categorical data and as means and standard deviations for continuous data. To examine the differences in characteristics according to periodontal disease risk, independent sample t‐tests (continuous data) and chi‐squared tests (categorical data) were undertaken.
To investigate the cross‐sectional associations between periodontal disease risk and single nutrient intakes, logistic regression analyses were undertaken. Single nutrient intakes, expressed as mean intakes per 1000 kcal of total energy, were divided into quartiles and entered into each model separately as the explanatory variable. Quartile 1 was entered as the reference category and represented the lowest intake. Periodontal disease risk status (≥1 symptoms vs. no symptoms) was the outcome variable in each model. The adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Confounders identified from the previous literature included age (years), sex (male/female), ethnicity (White/non‐White), TDI score, smoking status (currently/previous/never), alcohol drinking status (currently/previously/never), and BMI (kilogram per square metre).
Nutrient‐based dietary patterns were identified using a dimension reduction technique known as the principal component analysis (PCA). This data‐driven posteriori method statistically grouped the 20 nutrients, expressed as mean intakes per 1000 kcal of total energy, into a smaller number of uncorrelated underlying factors, also known as dietary patterns. The number of factors (dietary patterns) retained was based on the following criteria: eigenvalue >1, scree plot examination, and interpretability of the factors. Varimax rotation was performed to produce dietary patterns that were uncorrelated and interpretable. The dietary patterns were named according to dominant nutrients, which indicate having an absolute rotated factor loading ≥4.0 on a given factor (dietary pattern). Factor scores were calculated for each participant and divided into quartiles for each dietary pattern. Logistic regression analyses were carried out to investigate the cross‐sectional associations between periodontal disease risk status and each nutrient‐based dietary pattern. In each model, the nutrient‐based dietary pattern was the explanatory variable, with quartile 1 entered as the reference category and representing the lowest intake of that dietary pattern. Periodontal disease risk status (≥1 symptoms vs. no symptoms) was the outcome variable. All models were adjusted for age, sex, ethnicity, TDI score, smoking status, alcohol drinking status, and BMI (kilogram per square metre). The adjusted ORs and 95% CIs were calculated.
4. RESULTS
The characteristics of the total sample and by periodontal disease risk are presented in Table 1. Approximately 16% of the sample was aged 65 years or older, and over half the sample was classified as being overweight or obese (61%). Approximately 17% of the sample reported having one or more symptoms of periodontal disease.
TABLE 1.
Sample characteristics according to risk of periodontal disease
| Characteristic | Total (N = 9476), mean (SD) | Low risk of periodontal disease (n = 7842), mean (SD) | High risk of periodontal disease (n = 1634), mean (SD) | p‐Value |
|---|---|---|---|---|
| Socio‐demographic | ||||
| Sex (female) a , n (%) | 5421 (57.2) | 4381 (55.9) | 1041 (63.7) | <.001 |
| Age (years) | 56.18 (8.00) | 56.47 (7.99) | 54.79 (7.87) | <.001 |
| Ethnicity (White) a , n (%) | 9071 (95.7) | 7528 (95.9) | 1548 (94.7) | .021 |
| TDI score | −1.40 (2.72) | −1.47 (2.69) | −1.08 (2.88) | <.001 |
| Anthropometry | ||||
| Height (cm) | 169.41 (8.98) | 169.58 (8.98) | 168.57 (8.95) | <.001 |
| Weight (kg) | 77.03 (15.58) | 76.86 (15.39) | 77.88 (16.46) | .021 |
| BMI (kg/m2) | 26.76 (4.62) | 26.65 (4.53) | 27.33 (5.00) | <.001 |
| Health and dietary variables | ||||
| Smoking status a , n (%) | ||||
| Current | 621 (6.6) | 514 (6.6) | 108 (6.6) | |
| Previous | 3441 (36.3) | 2780 (35.4) | 662 (40.5) | |
| Never | 5414 (57.1) | 4552 (58.0) | 865 (52.9) | <.001 |
| Alcohol consumption a , n (%) | ||||
| Current | 8919 (94.1) | 7391 (94.2) | 1528 (93.5) | |
| Previous | 301 (3.2) | 237 (3.0) | 64 (3.9) | |
| Never | 261 (2.8) | 218 (2.8) | 43 (2.6) | .166 |
| Energy intake (kcal/day) | 2090.15 (501.93) | 2088.12 (499.81) | 2099.90 (512.02) | .388 |
Note: Continuous data are presented as mean (SD). Differences between periodontal disease risk groups were analysed using independent samples t‐tests for continuous variables and using chi‐squared test for categorical data.
Abbreviations: BMI, body mass index; TDI, Townsend Deprivation Index.
Categorical data are presented as frequencies (%).
Table 2 shows the unadjusted and adjusted ORs (95% CIs) for periodontal disease risk according to quartiles of single nutrient intakes. Higher intakes (Q2–Q4) compared with lower intakes (Q1, the reference category) of carbohydrate, dietary fibre, vitamins B6, B12, C, D, and E, folate, iron, potassium, magnesium, polyunsaturated fatty acids, and total sugar were significantly associated with reduced odds of having an increased risk of periodontal disease. Following adjustment for age, sex, ethnicity, TDI, BMI, and smoking and alcohol drinking status, significant associations remained for all the aforementioned nutrients with the exception of carbohydrate, dietary fibre, and vitamin D. An increased risk of periodontal disease was found for higher intakes of fat and saturated fat; however, after adjustment, significant associations remained only for higher intakes of saturated fat.
TABLE 2.
Odds ratios (OR) of periodontal disease risk and corresponding 95% confidence intervals (CIs) for nutrient intakes by quartile
| Nutrients (per 1000 kcal) | Q1 (lowest) | Q2 | Q3 | Q4 | |
|---|---|---|---|---|---|
| Ref. category | OR (95% CIs) | OR (95% CIs) | OR (95% CIs) | p‐Value for trend | |
| Protein (g/day) | — | 0.98 (0.84, 1.13) | 0.94 (0.81, 1.09) | 0.99 (0.85, 1.15) | .767 |
| — | 0.98 (0.84, 1.14) | 0.94 (0.81, 1.09) | 0.94 (0.81, 1.10) | .359 | |
| Carbohydrate (g/day) | — | 0.88 (0.76, 1.02) | 0.86 (0.74, 0.99) | 0.91 (0.78, 1.05) | .172 |
| — | 0.90 (0.77, 1.05) | 0.88 (0.75, 1.02) | 0.91 (0.78, 1.07) | .229 | |
| Starch (g/day) | — | 0.88 (0.75, 1.02) | 1.02 (0.88, 1.18) | 1.00 (0.86, 1.16) | .567 |
| — | 0.87 (0.75, 1.03) | 1.03 (0.88, 1.19) | 0.98 (0.85, 1.15) | .671 | |
| Total sugars (g/day) | — | 0.74 (0.64, 0.86) | 0.76 (0.65, 0.88) | 0.87 (0.75, 1.01) | .080 |
| — | 0.77 (0.66, 0.90) | 0.80 (0.68, 0.93) | 0.90 (0.78, 1.05) | .233 | |
| Fat (g/day) | — | 1.03 (0.88, 1.20) | 1.16 (0.99, 1.35) | 1.18 (1.01, 1.37) | .012 |
| — | 1.04 (0.89, 1.21) | 1.14 (0.98, 1.32) | 1.12 (0.96, 1.30) | .077 | |
| Saturated fat (g/day) | — | 1.14 (0.97, 1.33) | 1.22 (1.05, 1.42) | 1.24 (1.07, 1.45) | .003 |
| — | 1.13 (0.97, 1.32) | 1.19 (1.02, 1.39) | 1.20 (1.03, 1.41) | .016 | |
| Polyunsaturated FA (g/day) | — | 0.81 (0.70, 0.95) | 1.04 (0.89, 1.20) | 0.96 (0.82, 1.11) | .680 |
| — | 0.81 (0.69, 0.95) | 1.01 (0.87, 1.18) | 0.91 (0.79, 1.06) | .832 | |
| Dietary fibre (g/day) | — | 0.98 (0.84, 1.13) | 0.92 (0.80, 1.07) | 0.84 (0.73, 0.98) | .022 |
| — | 1.00 (0.86, 1.16) | 0.96 (0.82, 1.11) | 0.87 (0.74, 1.02) | .071 | |
| Retinol (μg/day) | — | 1.00 (0.86, 1.17) | 1.15 (0.99, 1.34) | 1.10 (0.94, 1.28) | .093 |
| — | 0.90 (0.77, 1.05) | 0.91 (0.78, 1.06) | 1.07 (0.92, 1.24) | .056 | |
| β‐Carotene (μg/day) | — | 1.10 (0.95, 1.28) | 1.01 (0.87, 1.18) | 1.03 (0.88, 1.20) | .243 |
| — | 0.95 (0.82, 1.11) | 0.97 (0.83, 1.13) | 0.90 (0.77, 1.05) | .237 | |
| Vitamin B6 (mg/day) | — | 0.86 (0.75, 1.00) | 0.84 (0.73, 0.98) | 0.80 (0.69, 0.93) | .003 |
| — | 0.91 (0.78, 1.05) | 0.88 (0.76, 1.02) | 0.83 (0.71, 0.96) | .015 | |
| Folate (μg/day) | — | 0.82 (0.70, 0.95) | 0.78 (0.68, 0.91) | 0.76 (0.65, 0.88) | <.001 |
| — | 0.86 (0.74, 1.00) | 0.83 (0.72, 0.97) | 0.81 (0.69, 0.94) | .005 | |
| Vitamin B12 (μg/day) | — | 0.91 (0.79, 1.06) | 0.85 (0.73, 0.99) | 0.80 (0.69, 0.93) | .002 |
| — | 0.94 (0.81, 1.09) | 0.89 (0.76, 1.03) | 0.83 (0.71, 0.97) | .011 | |
| Vitamin C (mg/day) | — | 0.81 (0.70, 0.94) | 0.80 (0.69, 0.93) | 0.76 (0.66, 0.89) | .001 |
| — | 0.83 (0.71, 0.96) | 0.81 (0.70, 0.94) | 0.77 (0.66, 0.90) | .001 | |
| Vitamin D (μg/day) | — | 0.97 (0.83, 1.12) | 0.97 (0.83, 1.12) | 0.86 (0.73, 1.00) | .057 |
| — | 1.00 (0.86, 1.16) | 1.00 (0.86, 1.16) | 0.89 (0.77, 1.04) | .175 | |
| Vitamin E (mg/day) | — | 0.99 (0.85, 1.15) | 0.90 (0.77, 1.05) | 0.85 (0.73, 0.99) | .018 |
| — | 0.97 (0.83, 1.13) | 0.87 (0.75, 1.02) | 0.80 (0.69, 0.94) | .003 | |
| Calcium (mg/day) | — | 1.00 (0.86, 1.17) | 1.10 (0.95, 1.28) | 0.97 (0.84, 1.13) | .601 |
| — | 1.12 (0.96, 1.30) | 0.99 (0.85, 1.16) | 0.99 (0.85, 1.16) | .560 | |
| Iron (mg/day) | 0.74 (0.64, 0.86) | 0.75 (0.65, 0.87) | 0.72 (0.62, 0.83) | <.001 | |
| — | 0.79 (0.68, 0.92) | 0.81 (0.70, 0.94) | 0.77 (0.66, 0.90) | .002 | |
| Potassium (mg/day) | — | 0.87 (0.75, 1.01) | 0.86 (0.74, 0.99) | 0.76 (0.65, 0.88) | <.001 |
| — | 0.92 (0.79, 1.07) | 0.91 (0.78, 1.06) | 0.79 (0.67, 0.92) | .004 | |
| Magnesium (mg/day) | — | 0.85 (0.73, 0.98) | 0.83 (0.72, 0.97) | 0.74 (0.63, 0.86) | <.001 |
| — | 0.88 (0.76, 1.02) | 0.88 (0.76, 1.02) | 0.78 (0.67, 0.91) | .003 |
Note: Data analysed using logistic regression. Data presented as unadjusted (top values) and adjusted OR and 95% CIs. Quartile (Q) 1 is the reference category and represents the lowest intake of a particular nutrient. Adjusted for age (years), sex (male/female), ethnicity (White/non‐White), Townsend Deprivation Index score, body mass index (kilogram per square metre), smoking status (never/previous/current), and alcohol drinking status (never/previous/current). Values highlighted in bold are significant (p < .05).
Abbreviation: FA, fatty acids.
Table 3 shows the factor loading matrix for the four major nutrient‐based dietary patterns identified by PCA. These four dietary patterns explained 64% of the variance and were named according to the nutrient loadings highest in each dietary pattern. The first dietary pattern was named “high micronutrient and fibre” and was characterized by the highest factor loadings for dietary fibre, β‐carotene, vitamins B6, C, and E, folate, iron, potassium, and magnesium. These nutrients would typically be found in fruits and vegetables. The second dietary pattern was named “high fat” and was characterized by high factor loadings for fat, saturated fat, and retinol. These nutrients would typically be found in high‐fat dairy products. The third pattern was named “high protein” and was characterized by high factor loadings for protein and vitamins B6, B12, and D. These nutrients would typically be found in meat, poultry, fish, and eggs. The fourth pattern was named “high sugar” and was characterized by high factor loadings for carbohydrates, total sugars, and calcium. These nutrients would typically be found in foods that contain high amounts of added sugars, such as biscuits, cakes, breakfast cereals, fruit yoghurt, and soda.
TABLE 3.
Factor loading matrix and explained variances for the four major nutrient‐based dietary patterns identified by principal component analysis
| Nutrient (per 1000 kcal) | Factor 1 (high micronutrient and fibre) | Factor 2 (high fat) | Factor 3 (high protein) | Factor 4 (high sugar) |
|---|---|---|---|---|
| Protein | 0.206 | −0.129 | 0.715 | −0.001 |
| Carbohydrate | 0.263 | −0.338 | −0.508 | 0.533 |
| Starch | −0.024 | −0.406 | −0.388 | −0.266 |
| Total sugars | 0.333 | −0.072 | −0.227 | 0.755 |
| Fat | −0.131 | 0.749 | 0.044 | −0.561 |
| Saturated fat | −0.291 | 0.834 | 0.025 | −0.067 |
| Polyunsaturated fat | 0.201 | 0.157 | −0.089 | −0.770 |
| Dietary fibre | 0.844 | −0.222 | −0.065 | −0.009 |
| Retinol | −0.084 | 0.766 | 0.041 | −0.047 |
| β‐Carotene | 0.733 | 0.018 | 0.070 | 0.049 |
| Vitamin B6 | 0.624 | −0.312 | 0.444 | 0.086 |
| Folate | 0.761 | −0.103 | 0.185 | 0.131 |
| Vitamin B12 | 0.061 | 0.059 | 0.821 | 0.043 |
| Vitamin C | 0.671 | −0.091 | 0.034 | 0.307 |
| Vitamin D | 0.103 | 0.087 | 0.694 | −0.083 |
| Vitamin E | 0.698 | 0.293 | −0.182 | −0.240 |
| Calcium | 0.322 | 0.255 | 0.150 | 0.520 |
| Iron | 0.541 | −0.441 | 0.277 | −0.073 |
| Potassium | 0.737 | −0.229 | 0.247 | 0.333 |
| Magnesium | 0.704 | −0.342 | 0.177 | 0.121 |
| Proportion of VAR explained (%) | 24.88 | 14.30 | 13.03 | 12.14 |
| Cumulative VAR explained (%) | 24.88 | 39.18 | 52.21 | 64.35 |
Note: Estimates from a principal component analysis carried out on 20 nutrients. Nutrients in bold have factor loadings ≥4.0 and have the same the dietary pattern.
Abbreviation: VAR, variance.
Table 4 shows the unadjusted and adjusted ORs and corresponding 95% CI for periodontal disease risk according to quartiles of nutrient‐based dietary patterns. The “high micronutrient and fibre” dietary pattern was inversely associated with having an increased risk of periodontal disease. This relationship remained following adjustment for age, sex, ethnicity, TDI, BMI, and smoking and alcohol drinking status (OR [95% CI] for highest intake [Q4] vs. lowest intake [Q1]: 0.76 [0.65–0.90]; p = .001). Higher intakes (Q3 and Q4) of the “high fat” dietary pattern compared with a lower intake (Q1, the reference category) were associated with an increased risk of periodontal disease. These associations did not remain after adjustment. No associations were observed for the “high sugar” or “high protein” nutrient‐based dietary patterns.
TABLE 4.
Odds ratios (OR) of periodontal disease risk and corresponding 95% confidence intervals (CIs) on quartiles of nutrient‐based dietary patterns identified by principal component analysis
| Nutrient‐based dietary patterns | Quartiles | ||||
|---|---|---|---|---|---|
| 1 (lowest) | 2 | 3 | 4 | ||
| Ref. category | OR (95% CI) | OR (95% CI) | OR (95% CI) | p‐Value for trend | |
| Factor 1: High micronutrient and fibre | — | 0.90 (0.77, 1.04) | 0.87 (0.75, 1.01) | 0.77 (0.66, 0.89) | .001 |
| — | 0.90 (0.77, 1.05) | 0.89 (0.77, 1.04) | 0.76 (0.65, 0.90) | .002 | |
| Factor 2: High fat | — | 1.12 (0.96, 1.31) | 1.20 (1.03, 1.40) | 1.17 (1.00, 1.36) | .029 |
| — | 1.12 (0.96, 1.31) | 1.16 (0.99, 1.35) | 1.13 (0.97, 1.32) | .119 | |
| Factor 3: High protein | — | 1.04 (0.89, 1.20) | 0.88 (0.76, 1.02) | 0.92 (0.79, 1.07) | .085 |
| — | 1.08 (0.93, 1.25) | 0.92 (0.79, 1.07) | 0.95 (0.81, 1.10) | .187 | |
| Factor 4: High sugars | — | 0.93 (0.80, 1.08) | 0.90 (0.77, 1.05) | 0.93 (0.80, 1.08) | .294 |
| — | 0.97 (0.84, 1.13) | 0.95 (0.82, 1.11) | 0.97 (0.84, 1.13) | .680 | |
Note: Data analysed using logistic regression. Data presented as unadjusted (top values) and adjusted OR and 95% CIs. Adjusted for age (years), sex (male/female), ethnicity (White/non‐White), Townsend Deprivation Index score, body mass index (kilogram per square metre), smoking status (never/previous/current), and alcohol drinking status (never/previous/current). Values highlighted in bold are significant (p < .05).
5. DISCUSSION
Within this sample of middle‐aged and older adults, higher intakes of vitamins B6, B12, C, and E, and folate, iron, potassium, and magnesium were significantly associated with a lower risk of periodontal disease, while higher intakes of saturated fat were associated with a higher risk of periodontal disease. These findings are similar to those of previous research in this area (O'Connor et al., 2020; Martinon et al., 2021). A relationship was observed in the current analysis between higher intakes of total sugar (second and third highest quartile intakes vs. lowest quartile intake) and lower periodontal disease risk. A high intake of sugar, however, has been previously associated with a higher risk of periodontal disease (O'Connor et al., 2020; Martinon et al., 2021). The total sugar variable used in the current analysis includes naturally occurring sugars found in many nutritious foods such as fruits, vegetables, and milk but also refined sugars. Separating the total sugar variable into the different sugar types may provide insight into this incongruous finding. The UK Biobank Resource, however, provided data for only 20 nutrients for the current analysis, which prevented further investigation.
A similar scenario was also observed for the polyunsaturated fatty acids variable, where the second lowest (quartile) intake was less likely than the lowest (quartile) intake to have a high risk of periodontal disease. The two highest intake groups (quartiles) were not associated with periodontal disease risk. Previous research has shown that omega‐3 fatty acids can have a beneficial effect on periodontal health, and an imbalance between omega‐6 and omega‐3 fatty acid intakes, where omega‐6 intakes are higher, may increase the risk of periodontal disease (Varela‐Lopez et al., 2016). Separation of the polyunsaturated fatty acid variable into omega‐3 and omega‐6 fatty acids would add value to the analysis and perhaps help explain the current result.
Four nutrient‐based dietary patterns were identified, with only one showing an association with risk of periodontal disease. This was the “high micronutrient and fibre” dietary pattern, which demonstrated higher intakes being associated with a lower risk of periodontal disease. Eight micronutrients including β‐carotene and dietary fibre had very high factor loadings in this dietary pattern. In the single nutrient analysis, however, dietary fibre and β‐carotene were not associated with periodontal disease risk following adjustment for socio‐demographic and health behaviour variables. This accentuates the significance of undertaking a dietary pattern analysis, as some nutrients may interact and in combination may be a stronger predictor of periodontal disease compared with when in isolation.
Although there is a lack of studies investigating the relationship between periodontal disease risk and posteriori nutrient‐based dietary patterns, the finding from the current analysis is in accordance to Wright et al.'s (2020) cross‐sectional analysis of the 2009–2014 NHANES data. They used the treelet transformation technique to identify dietary patterns based on food groups and then investigated the association with periodontitis. Their results showed that a dietary pattern rich in salads, fruits, vegetables, poultry, seafood, and plain water or tea was associated with a reduced extent of periodontitis. The “high micronutrient and fibre” dietary pattern identified in the current analysis likely represents a diet high in fruits and vegetables.
In contrast to Wright et al.'s (2020) and the current study's findings, a prospective study of 34,940 men enrolled in the Health Professionals Follow‐Up Study found over a 24‐year period no association between self‐reported incidence of periodontitis and a “prudent” dietary pattern characterized by high intakes of vegetables, fruits, legumes, whole grains, fish, and poultry (Alhassani et al., 2021). These conflicting findings may be due to different study designs, as Wright et al.'s (2020) was a cross‐sectional study, while Alhassani et al.'s (2021) was a longitudinal study and recruited only male participants. Furthermore, different periodontal disease measurements were used. In the NHANES study, a periodontal examination was carried out, which included the proportion of sites with clinical attachment ≥3 mm (Wright et al., 2020). In Alhassani et al. (2021) and in the current study, on the other hand, self‐reported measures were used. Currently, there are several different outcome definitions for periodontal disease used in epidemiology studies, which makes comparing the outcomes difficult in systematic reviews and prevents the pooling of data for meta‐analysis.
There were no associations observed for the other three nutrient‐based dietary patterns following adjustment for socio‐demographic and health behaviour variables. The “high fat” and “high sugar” dietary patterns would represent intakes typically consumed as part of a Western diet. Alhassani et al. (2021) found no association between periodontal disease and a “Western” dietary pattern characterized by a high intake of processed meat, red meat, butter, high‐fat dairy products, eggs, and refined grains. However, when a subgroup analysis of obese men in the same study was undertaken, an association was observed (Alhassani et al., 2021). Similar results were observed in other studies that have looked at specific food groups within the “Western” diet. Refined grains and a food group containing fish, shellfish, meat, beans, and eggs were not associated with periodontal disease (Merchant et al., 2006; Yoshihara et al., 2009). Dairy products and milk, however, have been shown to have a positive impact on periodontal disease (Al‐Zahrani, 2006; Lee & Kim, 2019).
The current analysis used self‐reported oral health information to estimate the risk of periodontal disease because a dental examination was not carried out. The self‐reported measure used can be subject to bias, resulting in an overestimation or underestimation of the true risk of periodontal disease in this population group. However, the surrogate markers used for the current analysis have demonstrated acceptable validity in place of a diagnosis of gingivitis/periodontitis made during a dental examination (Eke et al., 2013; Abbood et al., 2016). The markers have also been used by other UK Biobank studies (Jordão et al., 2019; Larvin et al., 2020, 2021; Lehrer et al., 2022). In the context of the current study, the term “periodontal disease risk” was used as a composite outcome to include both gingivitis and periodontitis. Self‐reported measures of oral health status are frequently used in large‐scale studies, as dental examinations can be expensive and time consuming. The oral health information collected by UK Biobank was limited, as no information was collected regarding oral hygiene, frequency of dental care, and the number of natural teeth. This information is essential for evaluating the overall oral health status and its association with general health and disease.
The 24‐h dietary recall method used can be subject to measurement error. Overestimation or underestimation of energy and nutrient intakes can occur. Recognized cut‐offs, however, were used in the current analysis to identify those participants with implausible energy intakes. Furthermore, the Oxford WebQ is a validated, web‐based, 24‐h dietary assessment tool (Greenwood et al., 2019) that has been used in other prospective studies such as the Million Women Study (Green et al., 2019). To capture habitual dietary intake and to reduce random error, the completion of multiple 24‐h recalls is required. Therefore, the participants were included in the current analysis only if they had completed at least two 24‐h assessments out of the five administered.
This is a cross‐sectional analysis, which makes it difficult to determine the direction of the association between dietary intake and periodontal disease risk. For instance, reduced periodontal support, discomfort, loose teeth, and tooth loss as a result of periodontitis may impair masticatory performance. Previous research has suggested that impaired masticatory ability can influence food selection and the consumption of important key nutrients, particularly in older adults (Watson et al., 2019). However, a recent systematic review that investigated the bidirectional relationship between dietary intake and periodontal health in community‐dwelling older adults found a lack of prospective studies providing evidence of the impact of periodontal disease on dietary intake; future studies are therefore warranted (O'Connor et al., 2020).
The data used for the analysis were collected over 10 years ago; it is therefore possible that food consumption, nutrient intakes, and nutritional status have changed over this time period. As a consequence, there is a risk that the findings are not generalizable to a more contemporary population group. Nevertheless, the strengths of this study include the large sample size (N = 9476), which is one of the larger studies investigating the association between periodontal disease and nutrient intakes (O'Connor et al., 2020). Furthermore, owing to the design of the UK Biobank study, with its main aim of investigating in depth the genetic and health information of a large sample of UK participants, a range of relevant data on potential confounding factors were made use of in the current study.
Although UK Biobank is not a representative sample of the UK population, as the volunteers were predominantly White and there is evidence of a “healthy volunteer effect” (Fry et al., 2017), the relationships between exposures and health conditions are thought to be generalizable to other populations due to the large sample size and the heterogeneity of exposure measures.
Finally, in common with all observational studies, the possibility of residual confounding or failure to account for other relevant confounders, such as stress or genetic predisposition, may have had some influence on the reported association between periodontal disease risk and dietary pattern. Additionally, our modelling did not take account of diabetic status as an independent risk factor for periodontal disease, as in the context of dietary pattern, hyperglycaemia is more likely to represent a mediator rather than a confounder for periodontal disease risk (Kocher et al., 2018). To explore diabetes as a mediator, repeated time measurements including a range of inflammatory biomarkers would be required. Recent work has highlighted the important mediation effect of other markers of systemic inflammation in relation to periodontitis, including oxidative stress (Sharma et al., 2021), platelet count (Romandini et al., 2018), and white blood cells (Torrungruang et al., 2018).
6. CONCLUSION
Within this sample of middle‐aged and older adults, a “high micronutrient and fibre” dietary pattern was associated with reduced risk of periodontal disease. Eight micronutrients including β‐carotene and dietary fibre had high factor loadings in this dietary pattern. In the single nutrient analysis, however, dietary fibre and β‐carotene were not associated with periodontal disease risk. This highlights the importance of undertaking a dietary pattern analysis, as some nutrients in combination may be a stronger predictor of periodontal disease. Currently, there are several different outcome definitions for periodontal disease used in epidemiological studies. Consensus regarding an appropriate periodontal disease diagnosis is therefore required to help facilitate future evidence synthesis. Futures studies investigating the relationship between dietary patterns and periodontal disease risk, especially of a longitudinal design, are warranted to help formulate dietary recommendations.
CONFLICT OF INTEREST
The authors declare no conflict of interest in connection with this article.
ETHICS STATEMENT
Full ethical approval for UK Biobank was obtained from the North West Haydock Research Ethics Committee. All participants provided informed consent to participation in UK Biobank.
AUTHOR CONTRIBUTIONS
All authors were involved in formulating the research question. Sinead Watson analysed the data. Sinead Watson wrote the manuscript, and all authors were involved in reading, revising it critically, editing, and approving the final manuscript.
ACKNOWLEDGEMENTS
This research has been conducted using data from UK Biobank, a major biomedical database, https://www.ukbiobank.ac.uk/ (Application number 27197). The Health and Social Care (HSC) Nutritional Translational Research Group financially supported this work.
Watson, S. , Woodside, J. V. , Winning, L. , Wright, D. M. , Srinivasan, M. , & McKenna, G. (2022). Associations between self‐reported periodontal disease and nutrient intakes and nutrient‐based dietary patterns in the UK Biobank. Journal of Clinical Periodontology, 49(5), 428–438. 10.1111/jcpe.13604
Funding information The Health and Social Care (HSC) Nutritional Translational Research Group
DATA AVAILABILITY STATEMENT
The anonymized datasets analysed during the current study are available at UK Biobank. Investigators may apply to access the UK Biobank study data through the processes described at http://www.ukbiobank.ac.uk/register-apply/.
REFERENCES
- Abbood, H. M. , Hinz, J. , Cherukara, G. , & Macfarlene, T. V. (2016). Validity of self‐reported periodontal disease: A systematic review and meta‐analysis. Journal of Periodontology, 87(12), 1474–1483. 10.1902/jop.2016.160196 [DOI] [PubMed] [Google Scholar]
- Alhassani, A. A. , Hu, F. B. , Li, Y. , Rosner, B. A. , Willett, W. C. , & Joshipura, K. J. (2021). The associations between major dietary patterns and risk of periodontitis. Journal of Clinical Periodontology, 48(1), 2–13. 10.1111/jcpe.13380 [DOI] [PubMed] [Google Scholar]
- Al‐Zahrani, M. S. (2006). Increased intake of dairy products is related to lower periodontitis prevalence. Journal of Periodontology, 77(2), 289–294. 10.1902/jop.2006.050082 [DOI] [PubMed] [Google Scholar]
- Al‐Zahrani, M. S. , Borawski, E. A. , & Bissada, N. F. (2005). Periodontitis and three health‐enhancing behaviors: Maintaining normal weight, engaging in recommended level of exercise, and consuming a high‐quality diet. Journal of Periodontology, 76(8), 1362–1366. 10.1902/jop.2005.76.8.1362 [DOI] [PubMed] [Google Scholar]
- Bawadi, H. A. , Khader, Y. S. , Haroun, T. F. , Al‐Omari, M. , & Tayyem, R. F. (2011). The association between periodontal disease, physical activity and healthy diet among adults in Jordan. Journal of Periodontal Research, 46(1), 74–81. 10.1111/j.1600-0765.2010.01314.x [DOI] [PubMed] [Google Scholar]
- Bernabe, E. , Marcenes, W. , Hernandez, C. R. , Bailey, J. , Abreu, L. G. , Alipour, V. , Amini, S. , Arabloo, J. , Arefi, Z. , Arora, A. , Ayanore, M. A. , Bärnighausen, T. W. , Bijani, A. , Cho, D. Y. , Chu, D. T. , Crowe, C. S. , Demoz, G. T. , Demsie, D. G. , Dibaji Forooshani, Z. S. , … Kassebaum, N. J. (2020). Global, regional, and national levels and trends in burden of oral conditions from 1990 to 2017: A systematic analysis for the global burden of disease 2017 study. Journal of Dental Research, 99(4), 362–373. 10.1177/0022034520908533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora, L. , Dubois, M. , Sacco, G. , & Lupi, L. (2021). Analysis the link between periodontal diseases and Alzheimer's disease: A systematic review. International Journal of Environmental Research and Public Health, 18(17), 9312. 10.3390/ijerph18179312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois, D. , Inquimbert, C. , Ottolenghi, L. , & Carrouel, F. (2019). Periodontal pathogens as risk factors for cardiovascular diseases, diabetes, rheumatoid arthritis, cancer, and chronic obstructive pulmonary disease – Is there cause for consideration? Microorganisms, 7(10), 424. 10.3390/microorganisms7100424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapple, I. L. C. , Genco, R. , & Working group 2 of joint EFP/AAP workshop . (2013). Diabetes and periodontal diseases: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systematic Diseases. Journal of Periodontology, 84, S106–S112. 10.1902/jop.2013.1340011 [DOI] [PubMed] [Google Scholar]
- Eke, P. I. , Dye, B. A. , Wei, L. , Slade, G. D. , Thornton‐Evans, G. O. , Beck, J. D. , Taylor, G. W. , Borgnakke, W. S. , Page, R. C. , & Genco, R. J. (2013). Self‐reported measures for surveillance of periodontitis. Journal of Dental Research, 92(11), 1041–1047. 10.1177/0022034513505621 [DOI] [PubMed] [Google Scholar]
- Fry, A. , Littlejohns, T. J. , Sudlow, C. , Doherty, N. , Adamska, L. , Sprosen, T. , Collins, R. , & Allen, N. E. (2017). Comparison of sociodemographic and health‐related characteristics of UKbiobank participants with those of the general population. American Journal of Epidemiology, 186(9), 1026–1034. 10.1093/aje/kwx246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gare, J. , Kanoute, A. , Meda, N. , Viennot, S. , Bourgeois, D. , & Carrouel, F. (2021). Periodontal conditions and pathogens associated with pre‐eclampsia: A scoping review. International Journal of Environmental Research and Public Health, 518(13), 7194. 10.3390/ijerph18137194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, J. , Reeves, G. K. , Floud, S. , Barnes, I. , Cairns, B. J. , Gathani, T. , Pirie, K. , Sweetland, S. , Yang, T. O. , Beral, V. , & Million Women Study Collaborators . (2019). Cohort profile: The million women study. International Journal of Epidemiology, 48(1), 28–29e. 10.1093/ije/dyy065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood, D. C. , Hardie, L. J. , Frost, G. S. , Alwan, N. A. , Bradbury, K. E. , Carter, M. , Elliott, P. , Evans, C. E. L. , Ford, H. E. , Hancock, N. , Key, T. J. , Liu, B. , Morris, M. A. , Mulla, U. Z. , Petropoulou, K. , Potter, G. D. M. , Riboli, E. , Young, H. , Wark, P. A. , & Cade, J. E. (2019). Validation of the Oxford WebQ online 24‐hour dietary questionnaire using biomarkers. American Journal of Epidemiology, 188(10), 1858–1867. 10.1093/aje/kwz165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, F. B. (2002). Dietary pattern analysis: A new direction in nutritional epidemiology. Current Opinion in Lipidology, 13(1), 3–9. 10.1097/00041433-200202000-00002 [DOI] [PubMed] [Google Scholar]
- Hujoel, P. P. , & Lingström, P. (2017). Nutrition, dental caries and periodontal disease: A narrative review. Journal of Clinical Periodontology, 44(18), S79–S84. 10.1111/jcpe.12672 [DOI] [PubMed] [Google Scholar]
- Jauhiainen, L. M. , Suominen, A. L. , Kanerva, N. , Männistö, S. , Knuuttila, M. , & Ylöstalo, P. V. (2016). Periodontal pocketing and gingival bleeding in relation to Nordic diet – Results from a population‐based survey. Journal of Clinical Periodontology, 43(12), 1013–1023. 10.1111/jcpe.12631 [DOI] [PubMed] [Google Scholar]
- Jauhiainen, L. M. , Ylöstalo, P. V. , Knuuttila, M. , Männistö, S. , Kanerva, N. , & Suominen, A. L. (2020). Poor diet predicts periodontal disease development in 11‐year follow‐up study. Community Dentistry and Oral Epidemiology, 48(2), 143–151. 10.1111/cdoe.12513 [DOI] [PubMed] [Google Scholar]
- Jordão, H. W. T. , McKenna, G. , McMenamin, U. C. , Kunzmann, A. T. , Murray, L. J. , & Coleman, H. G. (2019). The association between self‐reported poor oral health and gastrointestinal cancer risk in the UK Biobank: A large prospective cohort study. United European Gastroenterology Journal, 7(9), 1241–1249. 10.1177/2050640619858043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinane, D. F. , Stathopoulou, P. G. , & Papapanous, P. N. (2017). Periodontal diseases. Nature Reviews Disease Primers, 3, 17038. 10.1038/nrdp.2017.38 [DOI] [PubMed] [Google Scholar]
- Kocher, T. , König, J. , Borgnakke, W. S. , Pink, C. , & Meisel, P. (2018). Periodontal complications of hyperglycemia/diabetes mellitus: Epidemiologic complexity and clinical challenge. Periodontology 2000, 78(1), 59–97. 10.1111/prd.12235 [DOI] [PubMed] [Google Scholar]
- Larvin, H. , Kang, J. , Aggarwal, V. R. , Pavitt, S. , & Wu, J. (2021). Multimorbid disease trajectories for people with periodontitis. Journal of Clinical Periodontology, 48(12), 1587–1596. 10.1111/jcpe.13536 [DOI] [PubMed] [Google Scholar]
- Larvin, H. , Wilmott, S. , Wu, J. , & Kang, J. (2020). The impact of periodontal disease on hospital admissions and mortality during COVID‐19 pandemic. Frontiers in Medicine, 7, 604980. 10.3389/fmed.2020.604980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K. , & Kim, J. (2019). Dairy food consumption is inversely associated with the prevalence of periodontal disease in Korean adults. Nutrients, 11(5), 1035. 10.3390/nu11051035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer, S. , Rheinstein, P. H. , & Schmeidler, J. (2022). A component or multiple components of bleeding gums may ameliorate both glaucoma and Alzheimer's disease. Cureus, 14(1), e21004. 10.7759/cureus.21004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, A. , Chen, Y. , Schuller, A. A. , van der Sluis, L. W. M. , & Tjakkes, G. E. (2021). Dietary inflammatory potential is associated with poor periodontal health: A population‐based study. Journal of Clinical Periodontology, 48(7), 907–918. 10.1111/jcpe.13472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, B. , Young, H. , Crowe, F. J. , Benson, V. S. , Spencer, E. A. , Key, T. J. , Appleby, P. N. , & Beral, V. (2011). Development and evaluation of the Oxford WebQ, a low‐cost, web‐based method for assessment of previous 24 h dietary intakes in large‐scale prospective studies. Public Health Nutrition, 14, 1998–2005. 10.1017/S1368980011000942 [DOI] [PubMed] [Google Scholar]
- Maitre, Y. , Mahalli, R. , Micheneau, P. , Delpierre, A. , Amador, G. , & Denis, F. (2021). Evidence and therapeutic perspectives in the relationship between the oral microbiome and Alzheimer's disease: A systematic review. International Journal of Environmental Research and Public Health, 18(21), 11157. 10.3390/ijerph182111157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon, P. , Fraticelli, L. , Giboreau, A. , Dussart, C. , Bourgeois, D. , & Carrouel, F. (2021). Nutrition as a key modifiable factor for periodontitis and main chronic diseases. Journal of Clinical Medicine, 10(2), 197. 10.3390/jcm10020197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant, A. T. , Pitphat, W. , Franz, M. , & Joshipura, K. J. (2006). Whole‐grain and fiber intakes and periodontitis risk in men. The American Journal of Clinical Nutrition, 83(6), 1395–1400. 10.1093/ajcn/83.6.1395 [DOI] [PubMed] [Google Scholar]
- Nadim, R. , Tang, J. , Dilmohamed, A. , Yuan, S. , Wu, C. , Bakre, A. T. , Partridge, M. , Ni, J. , Copeland, J. R. , Anstey, K. J. , & Chen, R. (2020). Influence of periodontal disease on risk of dementia: A systematic literature review and a meta‐analysis. European Journal of Epidemiology, 35(9), 821–833. 10.1007/s10654-020-00648-x [DOI] [PubMed] [Google Scholar]
- O'Connor, J.‐L. P. , Milledge, K. L. , O'Leary, F. , Cumming, R. , Eberhard, J. , & Hirani, V. (2020). Poor dietary intake of nutrients and food groups are associated with increased risk of periodontal diseae among community‐dwelling older adults: A systematic literature review. Nutrition Reviews, 78(2), 175–188. 10.1093/nutrit/nuz035 [DOI] [PubMed] [Google Scholar]
- Romandini, M. , Laforí, A. , Romandini, P. , Baima, G. , & Cordaro, M. (2018). Periodontitis and platelet count: A new potential link with cardiovascular and other systemic inflammatory diseases. Journal of Clinical Periodontology, 45(11), 1299–1310. 10.1111/jcpe.13004 [DOI] [PubMed] [Google Scholar]
- Salazar, C. R. , Laniado, N. , Mossavar‐Rahmani, Y. , Borrell, L. N. , Qi, Q. , Sotres‐Alvarez, D. , Morse, D. E. , Singer, R. H. , Kaplan, R. C. , Badner, V. , & Lamster, I. B. (2018). Better‐quality diet is associated with lower odds of severe periodontitis in US Hispanics/Latinos. Journal of Clinical Periodontology, 45(7), 780–790. 10.1111/jcpe.12926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, P. , Fenton, A. , Dias, I. H. K. , Heaton, B. , Brown, C. L. R. , Sidhu, A. , Rahman, M. , Griffiths, H. R. , Cockwell, P. , Ferro, C. J. , Chapple, I. L. , & Dietrich, T. (2021). Oxidative stress links periodontal inflammation and renal function. Journal of Clinical Periodontology, 48, 357–367. 10.1111/jcpe.13414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonetti, M. S. , Jepsen, S. , Jin, L. , & Otomo‐Corgel, J. (2017). Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. Journal of Clinical Periodontology, 44(5), 456–462. 10.1111/jcpe.12732 [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S. , Van Dyke, T. E. , & Working group 1 of the joint EFP/AAP workshop . (2013). Periodontitis and atherosclerotic cardiovascular disease: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systematic Diseases. Journal of Clinical Periodontology, 40(14), S24–S29. 10.1111/jcpe.12089 [DOI] [PubMed] [Google Scholar]
- Torrungruang, K. , Ongphiphadhanakul, B. , Jitpakdeebordin, S. , & Sarujikumjornwatana, S. (2018). Mediation analysis of systemic inflammation on the association between periodontitis and glycaemic status. Journal of Clinical Periodontology, 45(5), 548–556. 10.1111/jcpe.12884 [DOI] [PubMed] [Google Scholar]
- UK Biobank . (2007). UK Biobank: Protocol for a large‐scale prospective epidemiological resource . https://www.ukbiobank.ac.uk/media/gnkeyh2q/study-rationale.pdf
- Varela‐Lopez, A. , Giampieri, F. , Bullon, P. , Battino, M. , & Quiles, J. L. (2016). Role of lipids in the onset, progression and treatment of periodontal disease. A systematic review of studies in humans. International Journal of Molecular Sciences, 17(8), 1202. 10.3390/ijms17081202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, S. , McGowan, L. , McCrum, L. A. , Cardwell, C. R. , McGuinness, B. , Moore, C. , Woodside, J. V. , & McKenna, G. (2019). The impact of dental status on perceived ability to eat certain foods and nutrient intakes in older adults: Cross‐sectional analysis of the UK National Diet and Nutrition Survey 2008‐2014. International Journal of Behavioral Nutrition and Physical Activity, 16(1), 43. 10.1186/s12966-019-0803-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, D. M. , McKenna, G. , Nugent, A. , Winning, L. , Linden, G. J. , & Woodside, J. V. (2020). Association between diet and periodontitis: A cross‐sectional study of 10,000 NHANES participants. The American Journal of Clinical Nutrition, 112(6), 1485–1491. 10.1093/ajcn/nqaa266 [DOI] [PubMed] [Google Scholar]
- Yoshihara, A. , Watanabe, R. , Hanada, N. , & Miyazaki, H. (2009). A longitudinal study of the relationship between diet intake and dental caries and periodontal disease in elderly Japanese subjects. Gerodontology, 26(2), 130–136. 10.1111/j.1741-2358.2008.00244.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The anonymized datasets analysed during the current study are available at UK Biobank. Investigators may apply to access the UK Biobank study data through the processes described at http://www.ukbiobank.ac.uk/register-apply/.


