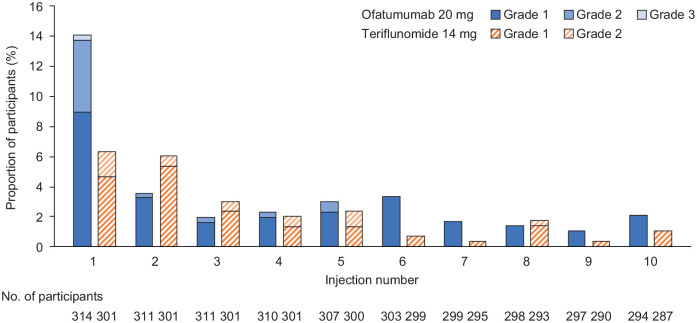
Figure 4.
Proportion of RDTN participants with injection-related systemic reactions following the first 10 injections in the study.
RDTN: recently diagnosed, treatment-naive.
Only Common Terminology Criteria for Adverse Events grades that were observed in the data are shown. Only reactions/symptoms occurring within 24 hours after injections are included (i.e. time to onset of reaction ⩽ 24 hours).
As teriflunomide is taken as an oral medication, injection-related systemic reactions in participants treated with teriflunomide are in response to placebo injections.