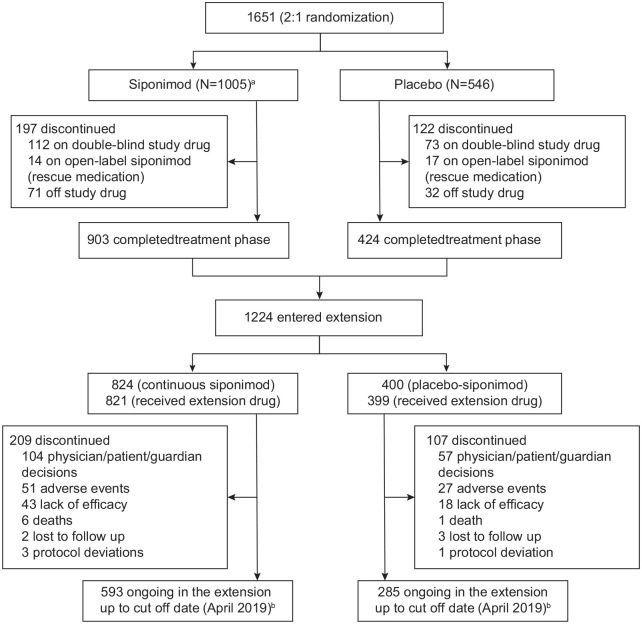
Figure 2.
Participant disposition (overall population).
Of the 1651 participants who were randomized (randomized set), 1646 received ⩾ 1 dose of randomized treatment (siponimod 2 mg or placebo) in the core part and were included in the analysis (full analysis set); 1224 participants entered the extension part; and 1220 received ⩾ 1 dose of open-label siponimod in the extension part.
a5 participants did not receive the study drug.bParticipants not included: those with a disposition reason that was not available in the database or an adverse event that occurred after cutoff date (6 April 2019) for 19 participants in the continuous siponimod group and 7 in the placebo-siponimod group.