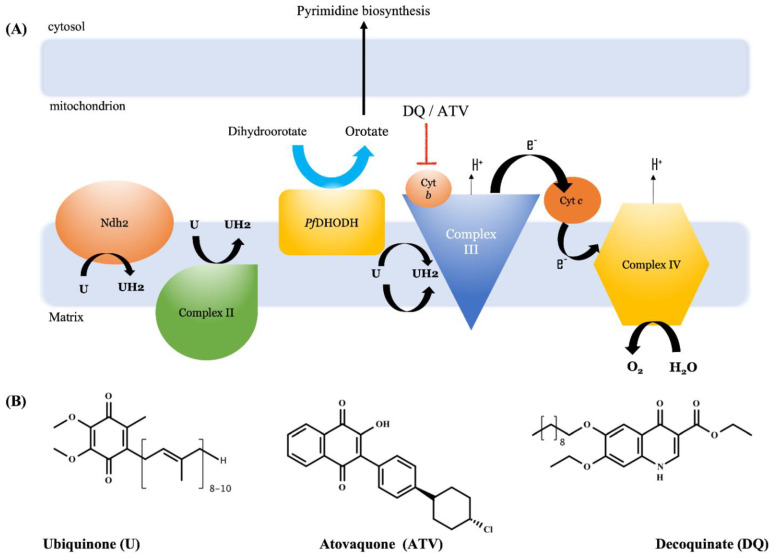
Figure 4.
Overview of pyrimidine metabolism in Plasmodium parasite and its drug blockage. Panel (A) shows that ubiquinone is recycled by redox chemistry from the dehydrogenase enzymes’ rotenone-insensitive internal NADH dehydrogenase (Ndh2) in complex I and P. falciparum dihydroorotate dehydrogenase (PfDHODH) in complex II. Complex III is involved in pyrimidine biosynthesis and regulation of the complex IV, which is necessary for the electron transport chain. Drugs that bind to the ubiquinone-binding site of cytochrome b of the bc1 cytochrome complex block the recovery of ubiquinone by PfDHODH and the transfer of electrons to downstream acceptors in the complex IV. This leads to a breakdown of the mitochondrial membrane potential and the subsequent death of the parasite. Panel (B) shows the chemical structures of ubiquinone (U) and the antiplasmodic drugs atovaquone and decoquinate, which compete with ubiquinone for the binding site in cytochrome b (Cyt b).