Abstract
Background:
Older adults admitted to an intensive care unit (ICU) are at risk of developing impairments in function, cognition, and mental health. It is not known whether socioeconomically disadvantaged older persons are at greater risk for these impairments than their less vulnerable counterparts.
Objective:
To evaluate the association between socioeconomic disadvantage and decline in function, cognition, and mental health among older survivors of an ICU hospitalization.
Design:
Retrospective analysis of a longitudinal cohort study.
Setting:
Community-dwelling older adults in the National Health and Aging Trends Study (NHATS).
Participants:
NHATS participants with ICU hospitalizations between 2011–2017.
Measurements:
Socioeconomic disadvantage was assessed as dual-eligible Medicare-Medicaid status. The outcome of function was defined as the count of disabilities in 7 activities of daily living and mobility tasks, the cognitive outcome as the transition from no or possible to probable dementia, and the mental health outcome as the Patient Health Questionnaire-4 (PHQ-4) score in the NHATS interview following ICU hospitalization. The analytic sample included 641 ICU hospitalizations for function, 458 for cognition, and 519 for mental health.
Results:
After accounting for sociodemographic and clinical characteristics, dual-eligibility was associated with a 28% increase in disability after ICU hospitalization (incidence rate ratio:1.28; 95% CI:1.00,1.64); and nearly 10-fold greater odds of transitioning to probable dementia (odds ratio:9.79; 95% CI:3.46,27.65). Dual-eligibility was not associated with symptoms of depression and anxiety following ICU hospitalization (incidence rate ratio:1.33; 95% CI: 0.99,1.79).
Limitations:
Administrative data, variability in timing of baseline and outcome assessments, proxy selection.
Conclusions:
Dual-eligible older persons are at greater risk of decline in function and cognition after an ICU hospitalization than their more advantaged counterparts. This finding highlights the need to prioritize low-income seniors in rehabilitation and recovery efforts after critical illness and warrants investigation into factors leading to this disparity.
Primary Funding Source:
National Institute of Aging
INTRODUCTION
New or worsening impairments in function, cognition, and mental health after a critical illness, described as the post-intensive care syndrome (PICS), present a mounting concern to patients, clinicians, and society (1–4). Older adults are especially susceptible to these impairments because of pre-existing factors such as frailty, cognitive impairment, and sensory deficits (5, 6). The number of older persons surviving an intensive care unit (ICU) stay annually in the United States (US), estimated at 1.4 million a decade ago (7), is expected to rise with the aging population (8, 9), improving survival following critical illness (10), and the current pandemic (11). Despite the growing impetus to improve recovery after critical illness, equity in patient-centered outcomes among ICU survivors has not been examined.
Disparities by race, insurance, and socioeconomic status that widely plague our healthcare system have been described in short-term mortality and readmissions from conditions such as pneumonia, sepsis, and acute respiratory failure (12–15). Socioeconomically disadvantaged persons age≥65 years who meet thresholds of low income and assets may qualify for Medicaid in addition to Medicare (16, 17). They are classified as “dual-eligible” for Medicare and Medicaid and known to have greater chronic disease burden and worse health outcomes for many conditions compared to non-dual-eligible Medicare beneficiaries (17–21). In a state-level study of ICU survivors, dual-eligibility was associated with a 9% greater risk of 1-year mortality compared to Medicare with supplemental insurance (22). However, it is not known whether dual-eligible seniors are at increased risk of impairments after ICU survivorship than non-dual-eligible beneficiaries.
Using a longitudinal study of Medicare beneficiaries with comprehensive, annual geriatric assessments, our objective was to evaluate whether socioeconomic disadvantage is associated with decline in function, cognition, and mental health following ICU hospitalization.
METHODS
Study population
Data were drawn from the National Health and Aging Trends Study (NHATS), a longitudinal, nationally representative survey of community-dwelling Medicare beneficiaries ages ≥65 living in the contiguous United States (23). The initial sample was drawn from Medicare enrollment database on September 30, 2010 with oversampling of non-Hispanic Blacks and the oldest age groups (24). The survey collected information on demographics, living arrangement, health conditions, disability, and cognitive status through annual in-person interviews starting in 2011. If a participant was not available for interview, a proxy knowledgeable about their health was interviewed. For participants who died between initial and follow-up rounds, a last month of life interview was conducted with the proxy. We used data from rounds 1–8 (2011–2018) for the 2011 cohort. The Johns Hopkins University Institutional Review Board approved the NHATS protocol and the Yale Institutional Review Board approved this study (HIC#1607018022). We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Supplement Table 1).
Ascertainment of ICU admissions and acquisition of ICU hospitalization data
ICU admissions were identified through linked Medicare fee-for-service inpatient claims files using critical care revenue codes indicating admission to general, specialty, or coronary care units, but excluding psychiatric and intermediate care units (25). Information on use of mechanical ventilation was obtained using International Classification of Diseases-9 CM and International Classification of Diseases-10 CM procedure codes (26). ICU length of stay was determined based on the days for which critical care was billed. Hospital length of stay, primary discharge diagnoses, and discharge disposition were extracted for each hospitalization.
Assessment of Function
During in-person interviews, participants or proxies were asked about need for help in activities of daily living including four self-care activities (eating, bathing, using the toilet, and dressing) and three mobility activities (getting outside, getting around inside one’s home, getting out of bed). Disability was characterized as the need for help or inability to perform these activities. For participants who died during follow-up, function was ascertained from the last month of life interview wherein the proxy was asked if in the last month of life, the participant needed help or was unable to complete the aforementioned activities. Our outcome for function was the count of disabilities on a scale of 0–7 assessed in the interview following discharge from ICU hospitalization (hereafter post-ICU interview) (27, 28). We used the count of disabilities in the interview preceding ICU hospitalization (hereafter pre-ICU interview) as the measure of baseline function.
Assessment of Cognition
We used the validated NHATS classification scheme for dementia status that defined “probable” dementia as one of the following: (a) self- or proxy-reported physician diagnosis of dementia; (b) score of ≥2 on the 8-item Alzheimer’s Disease-8 Dementia Screening interview of proxy respondents (29); or (c) scores of ≤1.5 standard deviations (SD) on ≥2 cognitive tests in the domains of memory (scale:0–20, cutoff ≤ 3), orientation (scale:0–8, cutoff ≤ 3), and executive function (scale:0–5, cutoff ≤ 1) (30, 31). SDs were derived from cognitive test scores of NHATS self-respondents (31). Scores of ≤1.5 SD in one domain were classified as “possible” dementia. A detailed description of cognitive assessment is provided in the supplement (Supplement Methods and Supplement Tables 2–4). Based on prior literature, we used the NHATS narrow dementia definition of probable vs no or possible dementia (32, 33) and defined our outcome as transition from no/possible dementia pre-ICU dementia to probable post-ICU dementia.
Assessment of Mental Health
Symptoms of depression and anxiety were assessed using the Patient Health Questionnaire for Depression and Anxiety (PHQ-4), which includes a depression subscale (PHQ-2) and an anxiety subscale [Generalized Anxiety Disorder (GAD-2)] (34, 35). PHQ-4 has excellent reliability and construct validity as a measure of depression and anxiety in the general population (34). NHATS participants were asked “over the last month, how often have you (a) had little interest or pleasure in doing things; (b) felt down, depressed, or hopeless; (c) felt nervous, anxious, or on edge; (d) been unable to stop or control worrying”. Each item was scored on a 4-point scale from “not at all”(0), “several days”(1), “more than half the days”(2) to “nearly every day”(3). Each subscale score ranges from 0–6, the total score ranging from 0–12. Our outcome for mental health was the post-ICU PHQ-4 score with pre-ICU PHQ-4 score as the baseline.
Ascertainment of dual-eligible status
Our primary exposure, dual-eligibility, was assessed using the dual Medicare-Medicaid status indicator in the Medicare Master Beneficiary Summary File, recorded at any time during the 12 months prior to ICU hospitalization.
Covariates
We carefully selected covariates for each outcome based on prior research and clinical relevance. Variables included in models for all outcomes were age categorized into five groups: 65–74, 75–79, 80–84, 85–89, and ≥90 years (36); sex (37), non-White race or ethnicity (non-Hispanic Blacks, Hispanics, American Indian, Alaska Native, Asian, Native Hawaiian, Pacific Islander, and other as self-reported in NHATS); living alone, less than a high school education (4, 38), multimorbidity (defined as ≥3 self-reported chronic conditions, of a possible 9) (39), mechanical ventilation (40, 41) as dichotomous variables; and hospital length of stay (LOS) as a continuous variable. In addition, we included the baseline status, i.e. count of disabilities, no or possible dementia, and PHQ-4 score during the pre-ICU interview for function, cognition, and mental health, respectively. Finally, we included risk factors for decline in specific outcome domains, specifically frailty (ordinal, scale of 0–5) for function,(5) and depression (dichotomous) for cognition (42). For function and mental health, we also added rural residence (vs urban) as a covariate. The model for cognitive decline did not converge when rural residence was included.
Assembly of the Analytic Sample
Assembly of the analytic sample for each outcome is presented in Figure 1. We restricted our sample to the first ICU hospitalization in the interval between annual NHATS interviews with an ICU length of stay of ≥1 day (n=1,500). After excluding participants who were not community-dwelling (n=433), admitted from a nursing home or spent ≥100 days in a nursing home between pre-ICU interview and hospitalization (n=70), and those with missing data on race (n=8), 989 ICU admissions remained for consideration. Of these, 332 ICU hospitalizations were excluded because of in-hospital death (n=106), discharge to hospice (n=56), follow-up interview completed >365 days after discharge (n=73) or missing the entire follow-up interview (n=97). Of the 657 ICU admissions where participants survived to discharge and had follow-up interviews, we excluded participants with maximal impairment at baseline in each outcome domain (i.e., the participant could not get any worse). This implied a pre-ICU count of disabilities of 7/7 for function (n=16), baseline dementia status of probable dementia for cognition (n=109), and pre-ICU PHQ-4 score 12/12 for mental health (n=6). Interviews completed by proxy because of participant death following hospital discharge were missing information on dementia status and PHQ-4 score and were excluded for cognition and mental health (n=90 and n=132, respectively).
Figure 1. Flow Diagram for Assembly of the Analytic Sample for all Three Outcomes.
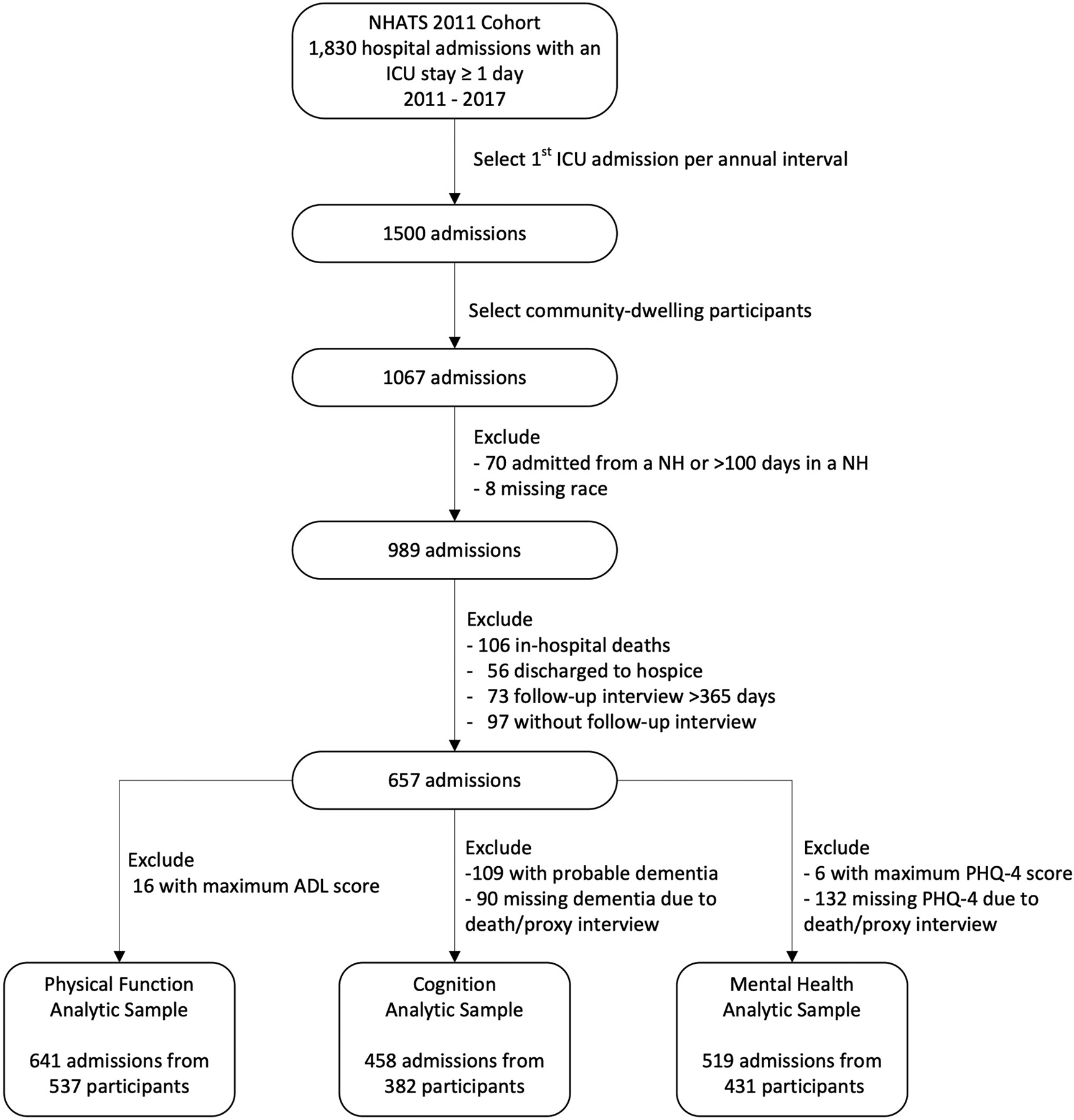
The sample included ICU hospitalizations of ≥ 1 day from participants enrolled in the NHATS 2011 cohort between the years of 2011–2017 to allow for follow-up assessment through 2018. Only the 1st ICU admission in the interval between two annual NHATS interviews for a participant (annual interval) was eligible for inclusion. After excluding participants who were admitted from a nursing home or spent ≥ 100 days in a nursing home between the pre-ICU interview and the ICU hospitalization, and those with missing data on race, 989 ICU admissions remained for consideration. Of these, 332 ICU hospitalizations were excluded because of in-hospital death (n=106), discharge to hospice (n=56), the follow-up interview occurring >365 days after discharge (n=73) or missing the entire follow-up interview (n=97). After excluding those with maximal impairment in each domain at pre-ICU interview and those with missing data because of participant death after hospital discharge, 641 admissions were eligible for the analysis of function, 458 admissions were eligible for the analysis of cognitive function, and 519 admissions for mental health. ICU = Intensive Care Unit, NH = Nursing Home.
Statistical Analysis
We first described clinical and demographic characteristics for each outcome by dual-eligibility status. For missing values of covariates and outcomes, we generated five imputations using PROC MI in SAS Version 9.4 based on an assumption of missing-at-random (Supplement Methods and Supplement Tables 5, 6). We fit multivariable models for the count outcomes of function and mental health using Poisson regression; for the binary indicator of cognitive decline we used multivariable logistic regression. We included the covariates described above. Covariates measured at the pre-ICU interview, such as age and multimorbidity, were included as time-constant variables. To account for variation in timing of hospitalization relative to pre and post ICU interviews, we included the number of days between hospital discharge and the post-ICU interview as an offset in the Poisson models. Unlike the Poisson models which can incorporate follow-up time as offset in model calculations, logistic regression models consider it as a separate covariate. The logistic regression model of cognitive decline did not converge with the addition of follow-up time as a covariate, thereby precluding its inclusion. We performed a sensitivity analysis with the time interval forced in as a covariate in the model with exclusion of some covariates in the main model.
NHATS is geographically clustered such that counties are sampled from regional strata across the U.S. Within these county-based clusters, persons of advanced older ages and Black race are oversampled from within zip codes to permit corresponding subgroup estimates. For each year of NHATS data, we used the specific analytic weights that adjust for differential probabilities of selection and non-response within each strata (region) and cluster (zip code within county), thereby allowing generalization to the 2011 Medicare population based on the information gathered from the individuals interviewed (43, 44). All models were fit using SAS-Callable SUDAAN Version 11(45) with weights, strata, cluster, and subpopulation statements that specified the eligible observations. For all models, there were instances where a minority of patients contributed multiple observations. To account for this, we used generalized estimating equations with an exchangeable covariance structure chosen by its minimization of quasi-likelihood under the independence model criterion. Within SUDAAN, all models were separately fit to each imputation with coefficients combined using Rubin’s rules as implemented in the LOGLINK (function and mental health) and RLOGIST (cognitive decline) procedures. For cognitive decline, we performed sensitivity analyses to assess for the robustness of our results to proxy-reporting and to competing risk of death. The latter involved imputation of only the missing outcomes among those participants whose follow-up was truncated by death under assumptions of missing at random and not missing at random (46). We also conducted sensitivity analyses to account for differences in indications for ICU hospitalization that might have different outcomes including 1) musculoskeletal conditions, 2) chronic diseases with waxing and waning courses such as chronic obstructive lung disease (COPD) and congestive heart failure (CHF), 3) stroke, and 4) acute neurologic conditions including traumatic brain injury, intracerebral hemorrhage, and status epilepticus. We further performed sensitivity analyses adjusting for additional factors including 1) ICU hospitalizations in the year prior to the index hospitalization, 2) rehospitalization to the ICU between hospitalization and post-ICU NHATS interview, 3) multimorbidity defined as ≥4 chronic conditions, 4) ICU LOS instead of hospital LOS, 5) census region, 6) type of ICU, and 7) discharge destination; and stratifying ICU admissions by 1) census region, 2) type of ICU admission, and 3) discharge destination. We additionally assessed for the robustness of our estimates to unmeasured confounding by calculating the E-value for outcomes with positive associations with dual-eligibility (47).
Role of the funding source:
This study was supported by funding from the National Institute of Aging which had no role in the study design, data analysis, or reporting.
RESULTS
Demographic and clinical characteristics for all hospitalizations based on dual-eligibility are presented in Table 1. Characteristics of the hospitalizations for the overall cohort for each outcome are described in Supplement Table 7. The mean age across the three samples ranged from 80.0 (SD:7.2) - 81.1 (SD:7.2) years. Distribution of the primary discharge diagnoses for the hospitalizations is presented in Supplement Table 8. Description of characteristics of hospitalizations of participants excluded because of absence of follow-up interviews or participant death is presented in Supplement Tables 9–11. Distribution of the timing between ICU hospitalization and pre- and post-ICU NHATS interviews is presented in Supplement Figures 1A–F. The majority (60.1%) of older ICU survivors were discharged to home (with or without services), 29.8% to skilled nursing or inpatient rehabilitation facilities, and 1.5% to long-term care hospitals.
Table 1.
Characteristics of ICU hospitalizations contributed by older adults for each outcome by dual-eligible status.
| Characteristic* | Function | Cognition | Mental Health | |||
|---|---|---|---|---|---|---|
| Dual-eligible | Non-dual-eligible | Dual-eligible | Non-dual-eligible | Dual-eligible | Non-dual-eligible | |
| Sample n | 130 | 511 | 80 | 378 | 108 | 411 |
| Weighted n | 658,095 | 3,109,600 | 409,991 | 2,381,242 | 544,373 | 2,574,141 |
| Age, mean (SD), years | 80.9 (7.7) | 81.0 (7.1) | 79.2 (7.7) | 80.2 (7.1) | 80.7 (8.0) | 80.4 (7.0) |
| Age groups by years, no. (%) | ||||||
| 65–74 | 27 (20.8) | 102 (20.0) | 22 (27.5) | 87 (23.0) | 25 (23.1) | 91 (22.1) |
| 75–79 | 28 (21.5) | 115 (22.5) | 22 (27.5) | 91 (24.1) | 24 (22.2) | 97 (23.6) |
| 80–84 | 34 (26.2) | 126 (24.7) | 17 (21.2) | 89 (23.5) | 25 (23.2) | 98 (23.8) |
| 85–89 | 22 (16.9) | 101 (19.8) | 10 (12.5) | 70 (18.5) | 16 (14.8) | 81 (19.7) |
| ≥ 90 | 19 (14.6) | 67 (13.1) | 9 (11.2) | 41 (10.8) | 18 (16.7) | 44 (10.7) |
| Female, no. (%) | 85 (65.4) | 244 (47.7) | 54 (67.5) | 192 (50.8) | 74 (68.5) | 202 (49.2) |
| Race, no. (%) | ||||||
| Non-Hispanic White | 56 (43.1) | 401 (78.5) | 36 (45.0) | 300 (79.4) | 44 (40.7) | 326 (79.3) |
| Non-Hispanic Black | 49 (37.7) | 85 (16.6) | 31 (38.8) | 62 (16.4) | 42 (38.9) | 69 (16.8) |
| Hispanic | 16 (12.3) | 13 (2.5) | 8 (10.0) | 8 (2.1) | 15 (13.9) | 8 (2.0) |
| Others† | 9 (6.9) | 12 (2.4) | 5 (6.2) | 8 (2.1) | 7 (6.5) | 8 (2.0) |
| Education, no. (%) | ||||||
| Less than high school | 77 (59.2) | 108 (21.2) | 43 (53.8) | 73 (19.3) | 63 (58.3) | 81 (19.7) |
| Living Situation, no. (%) | ||||||
| Lives alone | 61 (46.9) | 171 (33.5) | 45 (56.2) | 123 (32.6) | 51 (47.2) | 135 (32.9) |
| Rural residence, no. (%) | 45 (34.6) | 129 (25.2) | 29 (36.2) | 96 (25.4) | 38 (35.2) | 102 (24.8) |
| Multimorbidity ≥ 3 chronic conditions‡, no. (%) | 98 (75.4) | 332 (65.0) | 60 (75.0) | 240 (63.5) | 80 (74.1) | 263 (64.0) |
| Frequency of self-reported chronic conditions, no. (%) | ||||||
| Diabetes | 55 (42.6) | 176 (34.5) | 32 (40.5) | 137 (36.2) | 41 (38.3) | 146 (35.5) |
| Hypertension | 108 (83.1) | 409 (80.0) | 69 (86.2) | 302 (79.9) | 92 (85.2) | 328 (79.8) |
| Stroke | 14 (10.8) | 35 (6.9) | 7 (8.8) | 21 (5.6) | 13 (12.0) | 24 (5.8) |
| Heart disease | 64 (50.0) | 191 (37.8) | 39 (48.8) | 143 (38.0) | 51 (47.2) | 152 (37.2) |
| Arthritis | 108 (83.1) | 356 (69.7) | 66 (82.5) | 261 (69.0) | 89 (82.4) | 284 (69.1) |
| Heart attack | 23 (17.7) | 55 (10.8) | 15 (18.8) | 39 (10.3) | 20 (18.5) | 41 (10.0) |
| Osteoporosis | 41 (31.5) | 134 (26.2) | 28 (35.0) | 97 (25.7) | 37 (34.3) | 111 (27.0) |
| Lung disease | 42 (32.8) | 137 (26.8) | 25 (31.6) | 104 (27.5) | 31 (29.0) | 111 (27.0) |
| Non-skin cancer | 19 (14.7) | 99 (19.4) | 13 (16.2) | 71 (18.8) | 16 (14.8) | 75 (18.3) |
| Primary Discharge Diagnosis for Index Admission | ||||||
| Infectious | 20 (15.4) | 76 (14.9) | 12 (15.0) | 50 (13.2) | 19 (17.6) | 57 (13.9) |
| Endocrine, metabolic, or electrolyte disorders | 6 (4.6) | 8 (1.6) | 4 (5.0) | 7 (1.8) | 5 (4.6) | 7 (1.7) |
| Neurologic | 15 (11.5) | 51 (10.0) | 9 (11.2) | 37 (9.8) | 12 (11.1) | 44 (10.7) |
| Cardiovascular | 43 (33.1) | 217 (42.5) | 28 (35.0) | 173 (45.8) | 37 (34.3) | 183 (44.5) |
| Respiratory | 14 (10.8) | 33 (6.5) | 7 (8.8) | 18 (4.8) | 8 (7.4) | 19 (4.6) |
| Gastrointestinal | 15 (11.5) | 52 (10.2) | 12 (15.0) | 44 (11.6) | 13 (12.0) | 47 (11.4) |
| Renal | 6 (4.6) | 8 (1.6) | 1 (1.2) | 6 (1.6) | 4 (3.7) | 6 (1.5) |
| Musculoskeletal | 6 (4.6) | 29 (5.7) | 3 (3.8) | 21 (5.6) | 6 (5.6) | 23 (5.6) |
| Hematologic/oncologic | 5 (3.8) | 37 (7.2) | 4 (5.0) | 22 (5.8) | 4 (3.7) | 25 (6.1) |
| Frailty (Range 0–5), Median (IQR)¶ | 3.0 (2.0,4.0) | 2.0 (1.0,3.0) | 2.0 (1.0,3.0) | 1.0 (0.0, 2.0) | 3.0 (2.0,4.0) | 1.0 (1.0,2.0) |
| Pre-ICU count of disabilities**(Range 0–6), Median (IQR) | 1.0 (0.0,3.0) | 0.0 (0.0,1.0) | 0.0 (0.0,1.0) | 0.0 (0.0,0.0) | 1.0 (0.0,3.0) | 0.0 (0.0,1.0) |
| Pre-ICU PHQ-4†† (Range 0–11), Median (IQR) | 4.0 (1.0,6.0) | 1.0 (0.0,3.0) | 3.0 (1.0, 6.0) | 1.0 (0.0,3.0) | 3.5 (1.0,6.0) | 1.0 (0.0,3.0) |
| Pre-ICU PHQ-2 (Range 0–6)‡‡, Median (IQR) | 2.0 (0.0,3.0) | 0.0 (0.0,2.0) | 2.0 (0.0,3.0) | 0.0 (0.0,2.0) | 2.0 (0.0,3.0) | 0.0 (0.0,2.0) |
| Pre-ICU GAD-2 (Range 0–6)§§, Median (IQR) | 2.0 (0.0,3.0) | 0.0 (0.0,1.0) | 1.0 (0.0,3.0) | 0.0 (0.0,1.0) | 2.0 (0.0,3.0) | 0.0 (0.0,1.0) |
| Pre-ICU Dementia Status, no. (%) | ||||||
| No Dementia | 70 (53.8) | 387 (75.7) | 58 (72.5) | 319 (84.4) | 57 (52.8) | 317 (77.1) |
| Possible Dementia | 23 (17.7) | 67 (13.1) | 22 (27.5) | 59 (15.6) | 22 (20.4) | 59 (14.4) |
| Probable Dementia | 37 (28.5) | 57 (11.2) | 0 (0) | 0 (0) | 29 (26.8) | 35 (8.5) |
| Time interval between pre-ICU NHATS interview and ICU hospitalization, Median (IQR), days | 195.0 (115.0, 301.0) | 170.0 (93.0, 270.0) | 220.5 (137.0, 316.0) | 189.0 (110.0, 279.0) | 212.0 (128.5, 316.0) | 189.0 (109.0, 279.0) |
| ICU Length of Stay§, Median (IQR), days | 2.0 (1.0, 4.0) | 2.0 (1.0, 4.0) | 2.0 (1.0, 3.0) | 2.0 (1.0, 4.0) | 2.0 (1.0, 3.0) | 2.0 (1.0, 4.0) |
| Hospital Length of Stay§, Median (IQR), days | 6.0 (3.0,10.0) | 6.0 (3.0,9.0) | 6.0 (3.0,10.0) | 5.0 (3.0,8.0) | 6.0 (3.0,10.0) | 5.0 (3.0,8.0) |
| Mechanical ventilation∥, no. (%) | 11 (8.5) | 48 (9.4) | 4 (5.0) | 32 (8.5) | 6 (5.6) | 35 (8.5) |
| Time interval between ICU hospitalization and post-ICU NHATS interview, Median (IQR), days | 171.50 (85.0, 252.0) | 191.0 (108.0, 269.0) | 133.5 (71.0, 208.5) | 174.0 (97.0, 257.0) | 146.5 (68.0, 229.0) | 174.0 (98.0, 255.0) |
Abbreviations: ICU = Intensive Care Unit, SD = Standard Deviation, CI = Confidence Interval, IQR = Interquartile Range, PHQ-4 = 4-item screening questionnaire for depression and anxiety or Patient Health Questionnaire-4, PHQ-2 = 2-item screening questionnaire for Depression or Patient Health Questionnaire-2, GAD-2 = 2-item screening questionnaire for Generalized Anxiety Disorder. Pre-ICU refers to values obtained from the NHATS assessment prior to ICU hospitalization.
The unit of observation is ICU hospitalization.
Values represent characteristics for the unweighted sample.
Includes American Indian, Alaska Native, Asian, Native Hawaiian, Pacific Islander, and other race as self-reported in the NHATS interview.
Multimorbidity was defined as ≥ 3 of 9 self-reported chronic conditions (diabetes mellitus, hypertension, stroke, heart disease, arthritis, heart attack, osteoporosis, lung disease, and non-skin cancer)
Ascertained from hospitalization record in linked Medicare claims data.
Ascertained from linked Medicare claims data using ICD-9 CM (96.7x) and ICD-10-PCS (5A1935Z,5A1945Z,5A1955Z) codes for mechanical ventilation
The frailty score is derived from the composite of 1 point for each of the five frailty criteria (range 0–5)– weight loss, muscle weakness, exhaustion, slow gait speed, and low physical activity.
Count of disabilities was characterized as the need for help or inability to perform four activities of daily living (eating, bathing, using the toilet, and dressing) and three mobility activities (getting outside, getting around inside one’s home, getting out of bed). Participants with maximal score of 7/7 in the pre-ICU interview were excluded, hence the range from 0–6.
PHQ-4 score was the sum of the responses to all four items in the screening for depression and anxiety questionnaire, the measure for our outcome of mental health. Response for each question ranged from 0–3; the total score ranged from 0–12. Participants with maximal score of 12/12 in the pre-ICU interview were excluded.
PHQ-2 score was the sum of the responses to two items in the screening for depression questionnaire. Response for each question ranged from 0–3; the total score ranged from 0–6.
GAD-2 score was the sum of the responses to two items in the screening for anxiety questionnaire. Response for each question ranged from 0–3; the total score ranged from 0–6.
Function
We identified 641 participant-ICU stays representing 3,767,695 ICU hospitalizations after survey-weighting; 20.3% of these were contributed by dual-eligible participants. The median weighted post-ICU disability count was 2.18 (IQR:0.00,4.83) for dual-eligible participants and 0.01 (IQR:0.00,2.47) for non-dual-eligible participants. Figure 2 presents adjusted mean counts of post-ICU disability by pre-ICU disability and dual-eligibility. In the multivariable model, dual-eligibility was associated with a 28% greater post-ICU count of disability (incidence rate ratio:1.28; 95% CI:1.00,1.64). The E-value for this was 1.88 with a lower limit of confidence interval of 1.11.
Figure 2. Adjusted Summary Measures of Post-ICU Disability for Each Level of Pre-ICU Disability by Dual-Eligible Status.
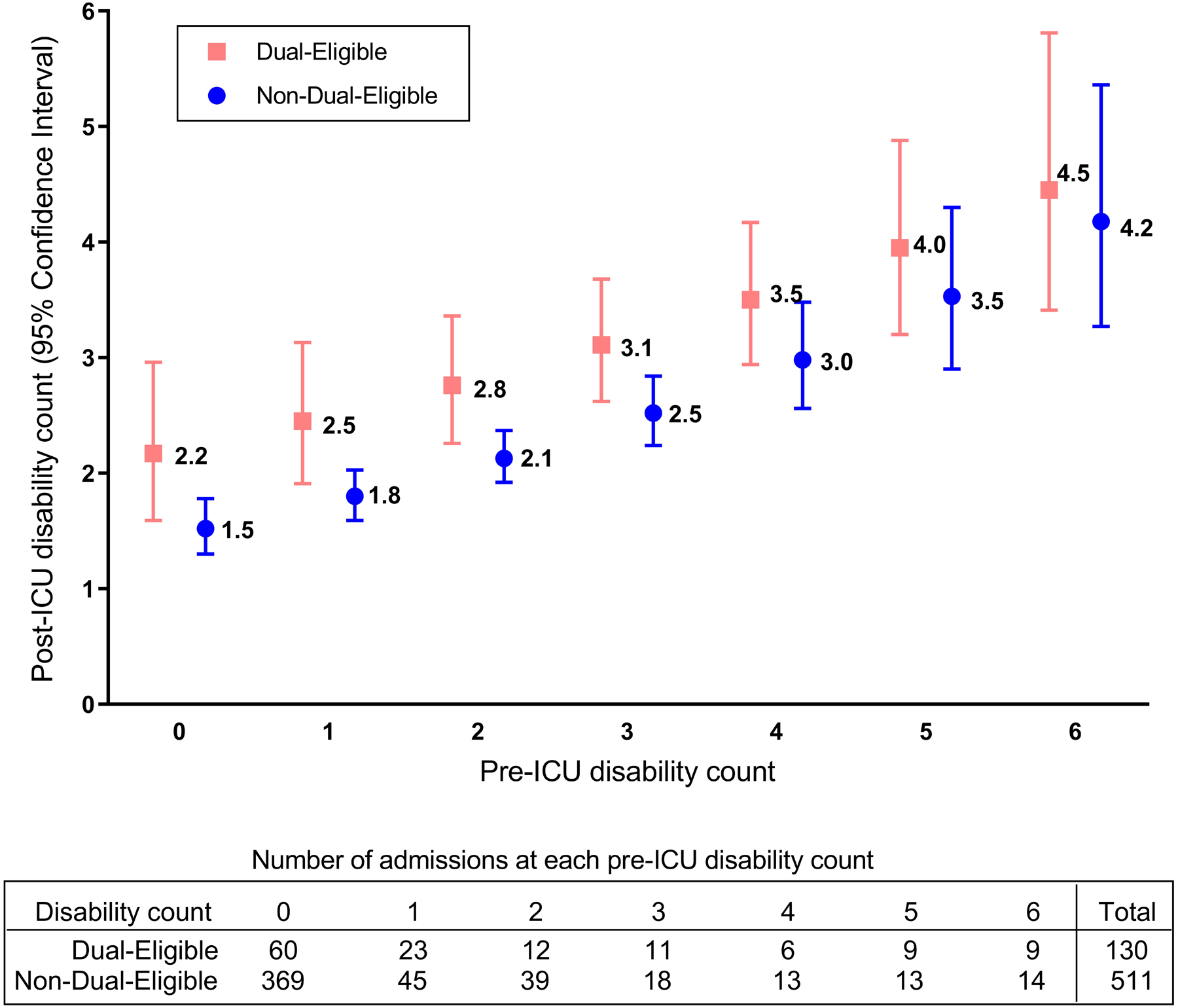
This figure shows the predicted marginal means of post-ICU count of disabilities, derived from the Poisson regression model after adjusting for all covariates including baseline (pre-ICU) count of disabilities and an offset for the time between discharge from ICU hospitalization and post-ICU interview. The point estimates present the mean post-ICU count of disabilities and the error bars represent the 95% CIs. The table presents the number of participant-ICU stays included in the calculation of these marginal means at each level of pre-ICU disability. ICU = Intensive Care Unit.
Cognition
We evaluated 458 participant-ICU stays representing 2,791,233 hospitalizations after survey-weighting; 14.7% were contributed by dual-eligible participants. 28% of dual-eligible participants had pre-ICU possible dementia compared to 16% of non-dual-eligible participants. 22.6% dual-eligible participants developed probable dementia after ICU hospitalization compared with 4.2% non-dual-eligible participants. Figure 3A presents the adjusted proportion of participants with post-ICU probable dementia by dual-eligible status. In the multivariable model, dual-eligibility was associated with 9.8 times greater odds of developing probable dementia (Odds Ratio: 9.79; 95% CI:3.46,27.65). The E-value for this was 19.1 with a lower limit of confidence interval of 6.38.
Figures 3A and 3B. Adjusted Proportion of Participants Developing Probable Dementia by Dual-Eligible Status (3A) and Adjusted Summary Measures of Post-ICU PHQ-4 Score by Dual-Eligible Status (3B).
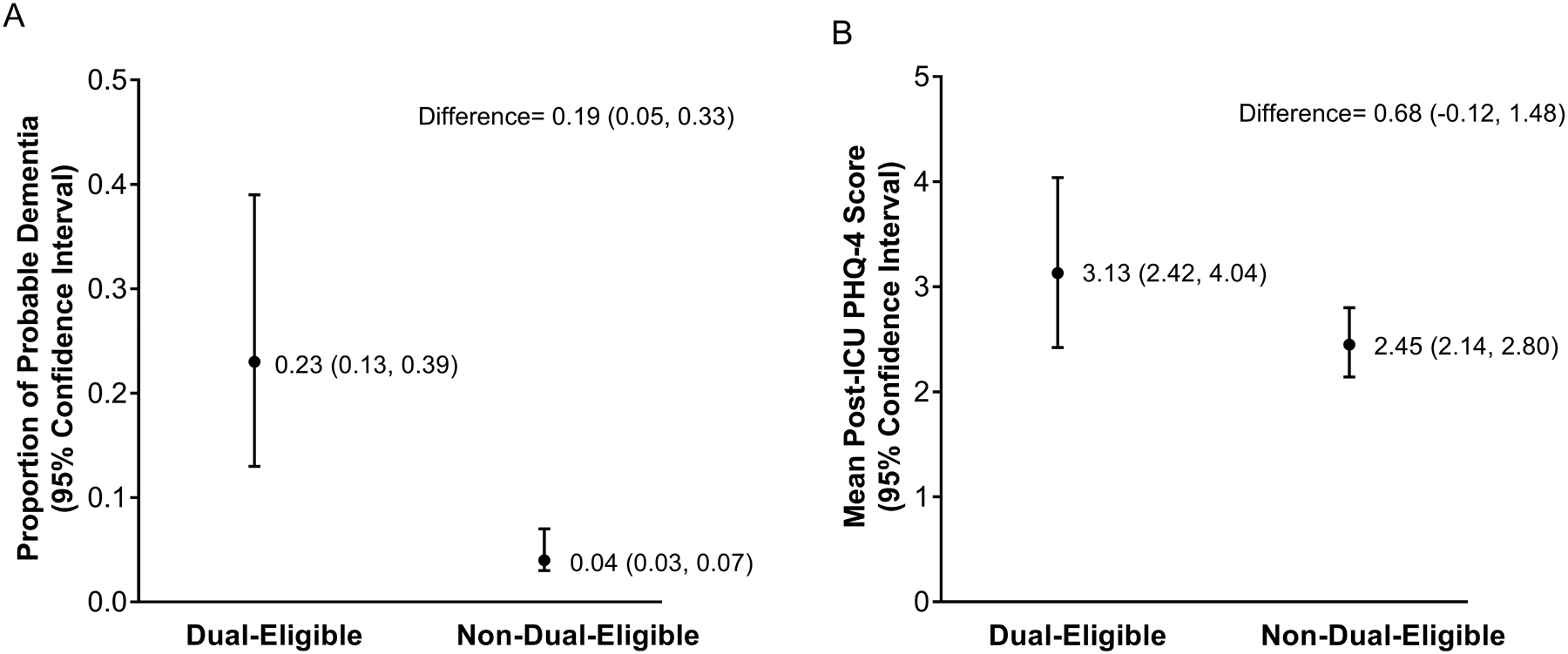
Figure 3A shows the predicted marginal proportions of participants who are expected to transition from no or possible dementia to probable dementia by dual-eligible status, derived from the logistic regression model after adjusting for all covariates including baseline possible dementia. The point estimates present the mean proportion of participants for dual-eligible and non-dual-eligible participants and the error bars represent the 95% CIs. Figure 3B presents the predicted marginal means of post-ICU PHQ-4 score (mental health outcome) by dual-eligible status, derived from the Poisson regression model after adjusting for all covariates including baseline (pre-ICU) PHQ-4 score and an offset for the time between discharge from ICU hospitalization and the post-ICU interview. The point estimates represent the mean PHQ-4 score for dual-eligible and non-dual-eligible participants and the error bars represent the 95% CIs. ICU = Intensive Care Unit, PHQ-4 = Patient Health Questionnaire-4.
Mental Health
Our analytic sample for mental health included 519 ICU hospitalizations, representing 3,118,513 hospitalizations; 17.5% of these were contributed by dual-eligible participants. Following ICU hospitalization, the median weighted PHQ-4 score was 3.51 (IQR:0.52, 6.09) among dual-eligible participants compared with 0.92 (IQR:0.0, 3.05) for non-dual-eligible participants. Figure 3B presents adjusted mean post-ICU PHQ-4 scores by dual-eligibility. In the multivariable model, dual-eligibility was not associated with post-ICU PHQ-4 score (incidence rate ratio:1.33, 95% CI:0.99,1.79). Pre-ICU PHQ-4 score was the only factor associated with post-ICU PHQ-4 score in this model (incidence rate ratio: 1.15, 95% CI: 1.11, 1.20, full model not shown).
Sensitivity Analyses
Results of all sensitivity analyses are presented in Appendix Tables 1–4. For the outcome of function, all of the sensitivity analyses did not substantially change the magnitude or direction of the association between dual-eligibility and post-ICU count of disabilities. For the outcome of cognition, the association between dual-eligibility and cognitive decline also remained similar in all analyses except in the extreme scenario wherein all decedents would have developed cognitive decline had they not died. For the outcome of mental health, the association between dual-eligibility and post-ICU PHQ-4 score did not change much in magnitude or direction but became statistically significant when excluding admissions with stroke, those with other acute neurologic conditions, when adding ICU type as a covariate, and when substituting ICU LOS for hospital LOS.
DISCUSSION
In this nationally representative study of community-dwelling Medicare beneficiaries with ICU hospitalizations, we found that socioeconomic disadvantage, represented by dual-eligible Medicaid status, was associated with a decline in function and cognition but not with symptoms of depression and anxiety following discharge. After accounting for known risk factors for functional decline including age, frailty, comorbidities, and pre-ICU disability, dual-eligible beneficiaries developed a nearly 30% greater burden of disability than their non-dual-eligible counterparts. After adjustment for confounders including age, education, and depressive symptoms, dual-eligible beneficiaries had nearly 10-fold greater odds of cognitive decline after ICU hospitalization than non-dual-eligible beneficiaries. In sensitivity analyses, the strong association between dual-eligibility and incident dementia was robust to the competing risk of death and to proxy-reporting, and to the exclusion of participants hospitalized with stroke. Post-ICU mental health symptoms, while worse among dual-eligible beneficiaries, were seemingly driven by pre-ICU mental health. Our finding that socioeconomically vulnerable older persons develop increased disability and dementia after ICU survivorship has meaningful implications for patients, families, healthcare systems, and policymakers.
To our knowledge, our study is the first to examine the association between individual-level socioeconomic disadvantage and all three domains of the post-ICU syndrome (PICS), which carries an enormous cost to patients and families (2, 48, 49). Prior studies outside the U.S. have reported an association between socioeconomic position and health-related quality of life but did not examine all three PICS domains (50). Two recent studies evaluating cohorts of middle-aged ICU survivors found education, but not neighborhood socioeconomic status, to be associated with decline in cognition and with decline in at least one domain of PICS, respectively (4, 38). Potential reasons for the differences in their findings could be their exclusion of participants with pre-existing disability, a younger population that may be less vulnerable to the development of deficits in the PICS domains, and use of neighborhood instead of individual measures of socioeconomic disadvantage (4, 38). Our study builds on these observations by examining the association of a widely recognized marker of individual-level socioeconomic disadvantage, dual-eligibility, which represents poverty among Medicare beneficiaries (17). Low-income adults in the US are more frequently exposed to adverse environments and have poorer access to high quality-healthcare compared to their higher income counterparts (17, 51). Our findings raise concern that socioeconomic vulnerability may be a major contributor to the differential development of functional and cognitive sequelae among ICU survivors.
Structural differences in hospital and post-acute care may also explain our findings. First, hospitals that care for the highest proportion of dual-eligible beneficiaries may provide lower quality of care, as has been described for acute myocardial infarction and heart failure (18, 20). Because adherence to early mobilization, delirium prevention, and other best practices in the hospital can affect downstream function and cognition, differences in their adoption may contribute to differences in long-term outcomes after discharge (52). Second, dual-eligible beneficiaries are often cared for in nursing homes and receive home health services from agencies with lower rankings, which could translate into less effective recovery (21, 53). Indeed, dual-eligible status has been associated with less improvement in disability among patients in home health care after hospitalization (54). Third, because of differences in coverage and payment policies between Medicare and Medicaid, dual-eligible beneficiaries are at risk for fragmented post-acute care (55). Finally, attendance and benefit from outpatient recovery programs may be limited among socioeconomically disadvantaged persons because of challenges with transportation (56), social support (57), and health literacy (58).
ICU hospitalizations for COPD and CHF seemed to contribute more to the observed socioeconomic differences in functional and cognitive decline as excluding these led to a reduction in the magnitude and/or loss of significance of the association between dual-eligibility and these outcomes. Since these are chronic conditions where disease outcomes may be influenced by access and adherence to treatment which are known to be worse for socioeconomically disadvantaged beneficiaries (20, 59), this could suggest a potential area of focus for clinicians and health systems. Sensitivity analyses excluding hospitalizations for acute neurologic conditions slightly increased the differences in post ICU PHQ-4. This may be explained by a more uniform effect of neurologic conditions on mental health for both dual-eligible and non-dual eligible participants such that excluding them unveils slightly greater disparities among those hospitalized with other conditions. Likewise, sensitivity analyses substituting ICU LOS rather than hospital LOS resulted in slightly increased differences in post ICU PHQ-4 and cognitive decline. Given the known association between depression and anxiety with prolonged lengths of stay in the ICU (60), slight increases in disparities when accounting for ICU LOS suggests that dual-eligibility may have a greater impact on cognition and mental health even with lower length of ICU stays.
Our findings have several implications. First, cognitive and functional decline among older persons is associated with institutionalization, mortality, and increased caregiver burden (61–63). The additional needs posed by decline in function and cognition can have devastating consequences for dual-eligible beneficiaries, who have greater baseline prevalence of dementia and disability, and lower levels of social and financial support than non-dual-eligible Medicare beneficiaries (17, 64–66). Second, since functional and cognitive impairment are known mediators of healthcare utilization for dual-eligible beneficiaries, worsening of these impairments following critical illness may contribute to hospital readmissions and further increase healthcare expenditures (67). An assessment of subsequent hospital readmissions and health expenditures would illuminate the impact of differences in functional and cognitive deficits among ICU survivors, however, was beyond the scope of our study. Third, an increase in disability and dementia after ICU survivorship can add to the need for long-term support services, potentially worsening the burden of unmet long term care needs for dual-eligible beneficiaries and increasing costs for Medicaid programs (19, 68). The downstream consequences of increased disability and dementia after ICU hospitalization can, therefore, worsen existing heath disparities for socioeconomically disadvantaged older adults.
A key strength of our study is the use of longitudinal comprehensive assessments of function, cognition, and mental health before and after hospitalization, allowing us to evaluate decline in all three PICS domains. Additionally, the availability of comprehensive geriatric assessments allowed us to adjust for important risk factors such as frailty. The use of a nationally representative sample, survey weighting, and oversampling of adults of minority race in NHATS further strengthens our study by allowing us to generalize our findings to the 2011 nationwide population of Medicare beneficiaries and to vulnerable populations.
Our study has several limitations. First, we linked the NHATS cohort to an administrative data source, which does not fully capture granular measures of severity of illness, delirium, hospital-stay related conditions, or treatment characteristics like use of early mobilization protocols (69, 70). Second, while dual-eligibility is a known proxy for socioeconomic disadvantage and is used extensively in studies of health outcomes including disability (21, 54), it does not distinguish individual social and economic risk factors. Third, because race is a social construct and tightly linked with socioeconomic disparities, the effects we observed cannot be considered independent of structural racism. Fourth, given the state-level variation in Medicaid eligibility criteria, our exposure could represent varying levels of economic disadvantage across this national cohort. Although we did not have information on state of residence, Medicaid eligibility in any state reflects low income with the median income limit for enrollment for older adults being 74% of Federal Poverty Limit (FPL), ranging from 63% to 100% of FPL across states (71). Fifth, we could not account for neighborhood geographic effects or hospital type due to unavailability of restricted use NHATS files that would allow linkages to these identifiers. These factors may be potential confounders as they are associated with life expectancy and socioeconomic status. However, we were able to account for broader geographic effects such as census region and rural status with the available data. Sixth, the timing of interviews in relation to hospitalizations was variable and could affect outcomes, particularly for outcomes that change over time; however, we accounted for this by including this timing as an offset in our models. Seventh, as is common in studies of older adults examining survival outcomes after critical illness (6), the final sample sizes for our outcome were reduced relative to the population admitted to ICUs. However, as detailed in the supplement, characteristics of participants included in our analyses were similar to those who were excluded because of missing outcome data or participant death. Eighth, because we excluded those with maximal impairments in each domain, we do not know if some of the participants with maximal impairments improved. However, prior work has demonstrated that severely disabled older adults either remain severely disabled or do not survive after a critical illness (40). Finally, since we restricted our sample to fee-for-service beneficiaries, our results may not be generalizable to those with managed Medicare plans.
CONCLUSIONS
In conclusion, we found that dual-eligible older persons are at greater risk of functional and cognitive decline following an ICU hospitalization. These findings highlight the need to prioritize low-income seniors in rehabilitation and recovery efforts after critical illness. Further research is needed to elucidate differences in acute and post-acute care that contribute to disparities in functional and cognitive decline after ICU survivorship.
Supplementary Material
ACKNOWLEDGEMENTS:
We acknowledge Dr. Thomas Gill for his contribution in acquiring and maintaining the NHATS data and guiding us in its use for this study.
GRANT SUPPORT:
Dr. Jain was funded by NIA T32AG1934. Dr. Ferrante was supported by NIA K76AG057023 and P30AG021342. Ms. Leo-Summers, Mr. O’Leary, and Dr. Murphy were supported by the Yale Claude D. Pepper Older Americans Independence Center (P30AG021342).
Footnotes
STATEMENT OF REPRODUCIBLE RESEARCH: The data source for the study was National Health and Aging Trends Study available from https://nhats.org/researcher/data-access. The authors are willing to share the study protocol and statistical code used for the analysis.
References:
- 1.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al. Long-Term Cognitive Impairment after Critical Illness. New England Journal of Medicine. 2013;369(14):1306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40(2):502–9. [DOI] [PubMed] [Google Scholar]
- 3.Iwashyna TJ. Survivorship Will Be the Defining Challenge of Critical Care in the 21st Century. Annals of Internal Medicine. 2010;153(3):204–5. [DOI] [PubMed] [Google Scholar]
- 4.Marra A, Pandharipande PP, Girard TD, Patel MB, Hughes CG, Jackson JC, et al. Co-Occurrence of Post-Intensive Care Syndrome Problems Among 406 Survivors of Critical Illness. Critical Care Medicine. 2018;46(9):1393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. The Association of Frailty With Post-ICU Disability, Nursing Home Admission, and Mortality A Longitudinal Study. Chest. 2018;153(6):1378–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Factors Associated with Functional Recovery among Older Intensive Care Unit Survivors. Am J Respir Crit Care Med. 2016;194(3):299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wunsch H, Guerra C, Barnato AE, Angus DC, Li G, Linde-Zwirble WT. Three-year outcomes for Medicare beneficiaries who survive intensive care. JAMA. 2010;303(9):849–56. [DOI] [PubMed] [Google Scholar]
- 8.Needham DM, Bronskill SE, Calinawan JR, Sibbald WJ, Pronovost PJ, Laupacis A. Projected incidence of mechanical ventilation in Ontario to 2026: Preparing for the aging baby boomers. Crit Care Med. 2005;33(3):574–9. [DOI] [PubMed] [Google Scholar]
- 9.Administration for Community Living. 2020 Profile of Older Americans. May 2021. Accessed June 26th, 2021. Available from https://acl.gov/sites/default/files/Aging%20and%20Disability%20in%20America/2020ProfileOlderAmericans.Final_.pdf.
- 10.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–54. [DOI] [PubMed] [Google Scholar]
- 11.Hosey MM, Needham DM. Survivorship after COVID-19 ICU stay. Nat Rev Dis Primers. 2020;6(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bime C, Poongkunran C, Borgstrom M, Natt B, Desai H, Parthasarathy S, et al. Racial Differences in Mortality from Severe Acute Respiratory Failure in the United States, 2008–2012. Annals of the American Thoracic Society. 2016;13(12):2184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soto GJ, Martin GS, Gong MN. Healthcare Disparities in Critical Illness. Critical Care Medicine. 2013;41(12):2784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar G, Taneja A, Majumdar T, Jacobs ER, Whittle J, Nanchal R, et al. The Association of Lacking Insurance With Outcomes of Severe Sepsis: Retrospective Analysis of an Administrative Database. Critical Care Medicine. 2014;42(3):583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnato AE, Alexander SL, Linde-Zwirble WT, Angus DC. Racial variation in the incidence, care, and outcomes of severe sepsis - Analysis of population, patient, and hospital characteristics. American Journal of Respiratory and Critical Care Medicine. 2008;177(3):279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Congressional Budget Office. Dual-Eligible Beneficiaries of Medicare and Medicaid: Characteristics, Health Care Spending, and Evolving Policies. 2013. Accessed at: https://www.cbo.gov/publication/44308. .
- 17.Medicare Payment Advisory Commission and the Medicaid and CHIP Payment and Access Commission. Data Book: Beneficiaries dually eligible for Medicare and Medicaid. 2018. Accessed April 30, 2021. Available from: http://medpac.gov/docs/default-source/data-book/jan18_medpac_macpac_dualsdatabook_sec.pdf?sfvrsn=0.
- 18.Lloren A, Liu S, Herrin J, Lin Z, Zhou G, Wang Y, et al. Measuring hospital-specific disparities by dual eligibility and race to reduce health inequities. Health Serv Res. 2019;54 Suppl 1:243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen SM, Piette ER, Mor V. The adverse consequences of unmet need among older persons living in the community: dual-eligible versus Medicare-only beneficiaries. J Gerontol B Psychol Sci Soc Sci. 2014;69 Suppl 1:S51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahiru E, Ziaeian B, Moucheraud C, Agarwal A, Xu H, Matsouaka RA, et al. Association of Dual Eligibility for Medicare and Medicaid With Heart Failure Quality and Outcomes Among Get With The Guidelines-Heart Failure Hospitals. JAMA Cardiol. 2021;6(7):791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joynt Maddox KE, Chen LM, Zuckerman R, Epstein AM. Association Between Race, Neighborhood, and Medicaid Enrollment and Outcomes in Medicare Home Health Care. J Am Geriatr Soc. 2018;66(2):239–46. [DOI] [PubMed] [Google Scholar]
- 22.Philpotts YF, Ma X, Anderson MR, Hua M, Baldwin MR. Health Insurance and Disparities in Mortality among Older Survivors of Critical Illness: A Population Study. J Am Geriatr Soc. 2019;67(12):2497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasper JD, Freedman VA. National Health and Aging Trends Study User Guide: Rounds 1–9 Final Release. Baltimore: Johns Hopkins University School of Public Health. 2020. Accessed April 30th, 2021. Available from www.NHATS.org. [Google Scholar]
- 24.Montaquila J, Freedman VA, Edwards B, Kasper JD. National Health and Aging Trends Study Round 1 Sample Design and Selection. NHATS Technical Paper #1. Baltimore: Johns Hopkins University School of Public Health. 2012. Accessed February 18th, 2021. Available from www.NHATS.org. [Google Scholar]
- 25.Kahn JM, Benson NM, Appleby D, Carson SS, Iwashyna TJ. Long-term acute care hospital utilization after critical illness. JAMA. 2010;303(22):2253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care. 2004;42(8):801–9. [DOI] [PubMed] [Google Scholar]
- 27.Ankuda CK, Ornstein KA, Covinsky KE, Boliens-Lund E, Meier DE, Kelley AS. Switching Between Medicare Advantage And Traditional Medicare Before And After The Onset Of Functional Disability. Health Affairs. 2020;39(5):809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falvey JR, Cohen AB, O’Leary JR, Leo-Summers L, Murphy TE, Ferrante LE. Association of Social Isolation With Disability Burden and 1-Year Mortality Among Older Adults With Critical Illness. JAMA Intern Med. 2021;181(11):1433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galvin JE, Roe CM, Powlishta KK, Coats MA, Muich SJ, Grant E, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559–64. [DOI] [PubMed] [Google Scholar]
- 30.Kasper JD, Freedman VA, Spillman BC, Skehan ME. Addendum to Classification of Persons by Dementia Status in the National Health and Aging Trends Study for Follow-up Rounds. Baltimore: Johns Hopkins University School of Public Health. 2015. Accessed April 30, 2021. Available from www.NHATS.org. [Google Scholar]
- 31.Kasper JD, Freedman VA, Spillman B. Classification of Persons by Dementia Status in the National Health and Aging Trends Study. Technical Paper #5. Baltimore: Johns Hopkins University School of Public Health. 2013. Accessed April 30, 2021. Available from www.NHATS.org. [Google Scholar]
- 32.Samuel LJ, Szanton SL, Wolff JL, Ornstein KA, Parker LJ, Gitlin LN. Socioeconomic disparities in six-year incident dementia in a nationally representative cohort of U.S. older adults: an examination of financial resources. BMC Geriatr. 2020;20(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freedman VA, Kasper JD, Spillman BC, Plassman BL. Short-Term Changes in the Prevalence of Probable Dementia: An Analysis of the 2011–2015 National Health and Aging Trends Study. J Gerontol B Psychol Sci Soc Sci. 2018;73(suppl_1):S48–S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowe B, Wahl I, Rose M, Spitzer C, Glaesmer H, Wingenfeld K, et al. A 4-item measure of depression and anxiety: validation and standardization of the Patient Health Questionnaire-4 (PHQ-4) in the general population. J Affect Disord. 2010;122(1–2):86–95. [DOI] [PubMed] [Google Scholar]
- 35.Kroenke K, Spitzer RL, Williams JB, Lowe B. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50(6):613–21. [DOI] [PubMed] [Google Scholar]
- 36.Herridge MS, Chu LM, Matte A, Tomlinson G, Chan LD, Thomas C, et al. The RECOVER Program: Disability. Risk Groups and 1-Year Outcome after 7 or More Days of Mechanical Ventilation. American Journal of Respiratory and Critical Care Medicine. 2016;194(7):831–44. [DOI] [PubMed] [Google Scholar]
- 37.Scheunemann LP, Leland NE, Perera S, Skidmore ER, Reynolds CF, Pandharipande PP, et al. Sex Disparities and Functional Outcomes after a Critical Illness. Am J Respir Crit Care Med. 2020;201(7):869–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haddad DN, Mart MF, Wang L, Lindsell CJ, Raman R, Nordness MF, et al. Socioeconomic Factors and Intensive Care Unit-Related Cognitive Impairment. Ann Surg. 2020;272(4):596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fortin M, Stewart M, Poitras ME, Almirall J, Maddocks H. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med. 2012;10(2):142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Functional trajectories among older persons before and after critical illness. JAMA Intern Med. 2015;175(4):523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barnato AE, Albert SM, Angus DC, Lave JR, Degenholtz HB. Disability among Elderly Survivors of Mechanical Ventilation. American Journal of Respiratory and Critical Care Medicine. 2011;183(8):1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jorm AF. Is depression a risk factor for dementia or cognitive decline? A review. Gerontology. 2000;46(4):219–27. [DOI] [PubMed] [Google Scholar]
- 43.Freedman VA, Hu M, DeMatteis J, Kasper JD. Accounting for Sample Design in NHATS and NSOC Analyses: Frequently Asked Questions. NHATS Technical Paper #23. Johns Hopkins University School of Public Health; 2020. [Google Scholar]
- 44.Montaquila J, Freedman Vicki A., Spillman Brenda, and Kasper Judith D. . National Health and Aging Trends Study Development of Round 1 Survey Weights. NHATS Technical Paper #2. . Baltimore: Johns Hopkins University School of Public Health. ; 2012. [Google Scholar]
- 45.Research Triangle Institute (2012). SUDAAN Language Manual, Volumes 1 and 2, Release 11. Research Triangle Park, NC. [Google Scholar]
- 46.Murphy TE, Gill TM, Leo-Summers LS, Gahbauer EA, Pisani MA, Ferrante LE. The Competing Risk of Death in Longitudinal Geriatric Outcomes. J Am Geriatr Soc. 2019;67(2):357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med. 2017;167(4):268–74. [DOI] [PubMed] [Google Scholar]
- 48.Kamdar BB, Suri R, Suchyta MR, Digrande KF, Sherwood KD, Colantuoni E, et al. Return to work after critical illness: a systematic review and meta-analysis. Thorax. 2020;75(1):17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davidson JE, Jones C, Bienvenu OJ. Family response to critical illness: postintensive care syndrome-family. Crit Care Med. 2012;40(2):618–24. [DOI] [PubMed] [Google Scholar]
- 50.Jones JRA, Berney S, Connolly B, Waterland JL, Denehy L, Griffith DM, et al. Socioeconomic Position and Health Outcomes Following Critical Illness: A Systematic Review. Crit Care Med. 2019;47(6):e512–e21. [DOI] [PubMed] [Google Scholar]
- 51.Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Aff (Millwood). 2002;21(2):60–76. [DOI] [PubMed] [Google Scholar]
- 52.Ely EW. The ABCDEF Bundle: Science and Philosophy of How ICU Liberation Serves Patients and Families. Crit Care Med. 2017;45(2):321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rahman M, Grabowski DC, Gozalo PL, Thomas KS, Mor V. Are dual eligibles admitted to poorer quality skilled nursing facilities? Health Serv Res. 2014;49(3):798–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chase JD, Huang L, Russell D, Hanlon A, O’Connor M, Robinson KM, et al. Racial/ethnic disparities in disability outcomes among post-acute home care patients. J Aging Health. 2018;30(9):1406–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grabowski DC. Medicare and Medicaid: conflicting incentives for long-term care. Milbank Q 2007;85(4):579–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chaiyachati KH, Hubbard RA, Yeager A, Mugo B, Shea JA, Rosin R, et al. Rideshare-Based Medical Transportation for Medicaid Patients and Primary Care Show Rates: A Difference-in-Difference Analysis of a Pilot Program. J Gen Intern Med. 2018;33(6):863–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jack K, McLean SM, Moffett JK, Gardiner E. Barriers to treatment adherence in physiotherapy outpatient clinics: a systematic review. Man Ther. 2010;15(3):220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levasseur M, Carrier A. Do rehabilitation professionals need to consider their clients’ health literacy for effective practice? Clin Rehabil. 2010;24(8):756–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spitzer KA, Stefan MS, Priya A, Pack QR, Pekow PS, Lagu T, et al. Participation in Pulmonary Rehabilitation after Hospitalization for Chronic Obstructive Pulmonary Disease among Medicare Beneficiaries. Ann Am Thorac Soc. 2019;16(1):99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davydow DS, Desai SV, Needham DM, Bienvenu OJ. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: A systematic review. Psychosomatic Medicine. 2008;70(4):512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc. 1997;45(1):92–100. [DOI] [PubMed] [Google Scholar]
- 62.Freedman VA, Spillman BC. Disability and care needs among older Americans. Milbank Q 2014;92(3):509–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boyd CM, Ricks M, Fried LP, Guralnik JM, Xue QL, Xia J, et al. Functional decline and recovery of activities of daily living in hospitalized, disabled older women: the Women’s Health and Aging Study I. J Am Geriatr Soc. 2009;57(10):1757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garfield R, Young K, Musumeci M, Reaves EL, Kasper JD. Serving Low-Income Seniors Where They Live: Medicaid’s Role in Providing Community-Based Long-Term Services and Supports. The Kaiser Commission on Medicaid and the Uninsured. 2015. Accessed March 29, 2021. Available from: https://www.kff.org/medicaid/issue-brief/serving-low-income-seniors-where-they-live-medicaids-role-in-providing-community-based-long-term-services-and-supports/view/footnotes/. [Google Scholar]
- 65.Nicholson NR. A review of social isolation: an important but underassessed condition in older adults. J Prim Prev. 2012;33(2–3):137–52. [DOI] [PubMed] [Google Scholar]
- 66.LaPlante MP, Kaye HS, Kang T, Harrington C. Unmet need for personal assistance services: estimating the shortfall in hours of help and adverse consequences. J Gerontol B Psychol Sci Soc Sci. 2004;59(2):S98–S108. [DOI] [PubMed] [Google Scholar]
- 67.Johnston KJ, Joynt Maddox KE. The Role Of Social, Cognitive, And Functional Risk Factors In Medicare Spending For Dual And Nondual Enrollees. Health Aff (Millwood). 2019;38(4):569–76. [DOI] [PubMed] [Google Scholar]
- 68.Komisar HL, Feder J, Kasper JD. Unmet long-term care needs: an analysis of Medicare-Medicaid dual eligibles. Inquiry. 2005;42(2):171–82. [DOI] [PubMed] [Google Scholar]
- 69.Kim DH, Lee J, Kim CA, Huybrechts KF, Bateman BT, Patorno E, et al. Evaluation of algorithms to identify delirium in administrative claims and drug utilization database. Pharmacoepidemiol Drug Saf. 2017;26(8):945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Israni J, Lesser A, Kent T, Ko K. Delirium as a predictor of mortality in US Medicare beneficiaries discharged from the emergency department: a national claims-level analysis up to 12 months. BMJ Open. 2018;8(5):e021258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Musumeci MB CP, Watts MO. Medicaid Financial Eligibility for Seniors and People with Disabilities: Findings from a 50-State Survey. 2019:Available at: https://www.kff.org/medicaid/issue-brief/medicaid-financial-eligibility-for-seniors-and-people-with-disabilities-findings-from-a-50-state-survey/. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


