Figure 7.
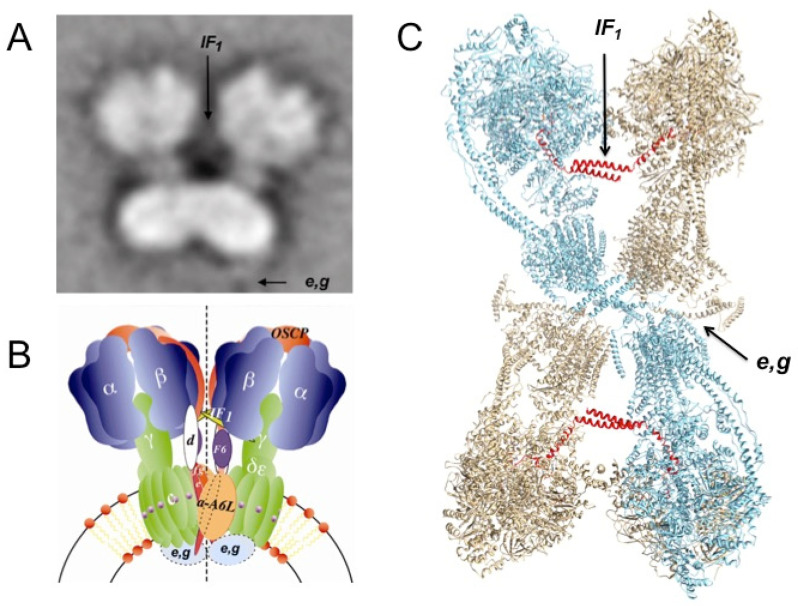
Double function of mitochondrial IF1as intrinsic F1FO-ATPase inhibitor and as dimerizing and oligomerizing factor of the mitochondrial F-ATP synthase. (A) The first EM 2D structure of bovine mitochondrial dimeric F1FO-IF1 complex [102] is shown as an average of the complete set of collected images, indicating the originally proposed sites for IF1 as a central protein bridge structure connecting the F1–F1 moieties. On the other hand, the proposed dimerizing subunits e and g form a “hanging bridge” at the Fo-Fo dimer interface [102]. (B) Two-dimensional cartoon model of the mitochondrial F-ATP synthase dimer showing the proposed positions of IF1 (yellow) and e and g subunits (red/blue) according to functional and mutagenesis studies [103,104,105,106,107]. (C) The structure of tetrameric mitochondrial F-ATP synthase of pig Sus scrofa as resolved by Cryo-EM depicted as two small-angle (≈40°) V-shaped homodimers bridged by an antiparallel coiled-coil homodimer of the IF1 inhibitor (red). Two large-angle dimers (≈60°–90°) form another, more open V-shaped dimer connected through FO-FO dimerizing subunits (e, g, and others) at the center of the tetramer. The dimerizing and oligomerizing function of mitochondrial IF1 lies on its C-terminal domain forming an antiparallel IF1-IF1 coiled-coil (red F1-F1 bridge and see Figure 1D and Figure 7), and its inhibitory domain is inserted in the αDPβDPγ interface of each monomer (see Figure 1C). The dimerizing function of IF1 was also demonstrated functionally by some of us with removal and reconstitution of IF1, which promoted, respectively, monomerization and dimerization/oligomerization of the mitochondrial rat liver F-ATP synthase. See Ref. [108] and text for further details.