Figure 2.
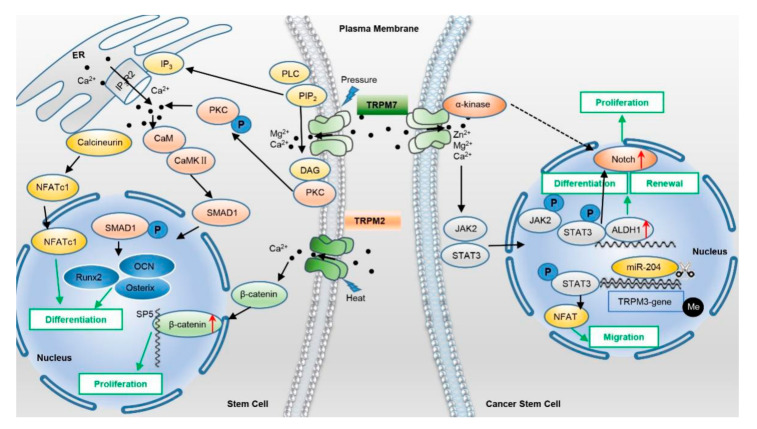
TRPMs function in stem cells and cancer stem cells via different signaling pathways. In MSCs, the Ca2+ influx via TRPM7 triggers the activation of the PLC signaling pathway, subsequently releases Ca2+ from ER, and induces nuclear translocation of NFATc1, promoting differentiation in human bone marrow MSCs. Moreover, activation of PLC leads to phosphorylation of PKC and its intracellular translocation, recruitment of CaM, and stimulation of CaMKII, which then phosphorylates SMAD1 and induces its translocation to the nucleus, thereby activating osteogenic genes Runx2, Osterix, and OCN to promote MSC differentiation and osteogenesis. Activation of TRPM2 by heat stress elevates intracellular calcium influx, which inhibits the phosphorylation of β-catenin and results in the augment of β-catenin on the SP5 promoter, promoting NPC proliferation. In GSCs, activation of TRPM7 activates the JAK2-STAT3 signaling pathway, which phosphorylates JAK2 and STAT3 and induces the translocation of JAK2 and STAT3 to the nucleus; STAT3 then activates ALDH1 via binding to its promoter and/or activates Notch signaling, enhancing GSC differentiation, renewal, and proliferation. Downregulation of miR-204 via methylation of the promoter of its host gene TRPM3 could activate the STAT3-NFAT pathway, promoting GSC invasion and stem cell-like phenotype.