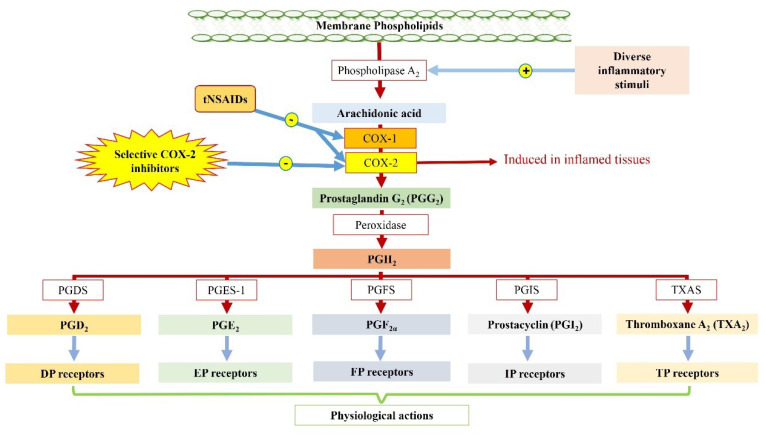
Figure 1.
Biosynthesis of prostaglandins and associated compounds. For instance, arachidonic acid is liberated by Ca2+ stimulation mediated by diverse inflammatory stimuli or phosphorylation of phospholipase A2 from the membrane phospholipid. The cyclooxygenase enzymes (both COX-1 and COX-2) epoxygenate arachidonic acid into prostaglandin G2 (PGG2), which is further converted into prostaglandin H2 (PGH2) with the help of peroxidase enzyme. Following this, PGH2 undergoes conversion into prostaglandin (PG) analogues (i.e., PGD2, PGE2, PGF2α, and PGI2 or prostacyclin) and thromboxane A2 (TxA2) by the help of isomerases and synthases, respectively. The formed PGs and associated compounds are capable of triggering a myriad of signalling events by activating their respective membrane receptors located at the site of production. The traditional non-steroidal anti-inflammatory drugs (tNSAIDs) inhibit cyclooxygenase enzyme non-selectively and prevent prostaglandin synthesis, whereas selective COX-2 inhibitors (coxibs) inhibit the COX-2 isoform that is induced by inflammation. PGDS: prostaglandin D synthase, PGES-1: prostaglandin E synthase-1, PGFS: prostaglandin F2α synthase, PGIS: prostacyclin synthase, and TXAS: thromboxane A2 synthase.