Abstract
T-cell intracellular antigen 1 (TIA1)-related/like (TIAR/TIAL1) protein is a multifunctional RNA-binding protein (RBP) involved in regulating many aspects of gene expression, independently or in combination with its paralog TIA1. TIAR was first described in 1992 by Paul Anderson’s lab in relation to the development of a cell death phenotype in immune system cells, as it possesses nucleolytic activity against cytotoxic lymphocyte target cells. Similar to TIA1, it is characterized by a subcellular nucleo-cytoplasmic localization and ubiquitous expression in the cells of different tissues of higher organisms. In this paper, we review the relevant structural and functional information available about TIAR from a triple perspective (molecular, cellular and pathophysiological), paying special attention to its expression and regulation in cellular events and processes linked to human pathophysiology.
Keywords: TIAR, TIAL1, gene expression, cellular homeostasis, pathophysiology
1. Introduction
TIAR: One Gene, Two Main Isoforms and a Classical RBP Structure
T-cell intracellular antigen 1-related/like (TIAR/TIAL1) protein is a classical member of the RNA-binding protein (RBP) family. It was first identified in 1992 by Paul Anderson and colleagues [1], and was named TIA1-related or TIA1-like protein due to its high degree of identity and structural homology with its paralog TIA1—which had been identified one year earlier by the same group [2].
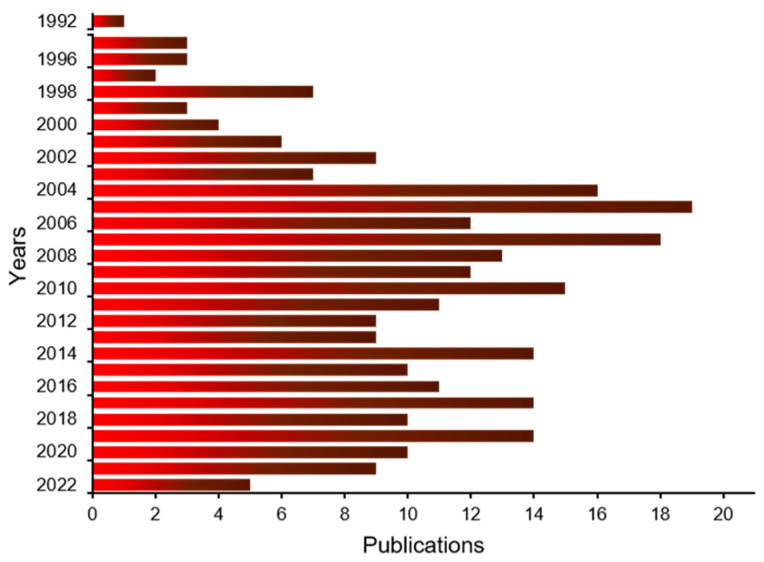
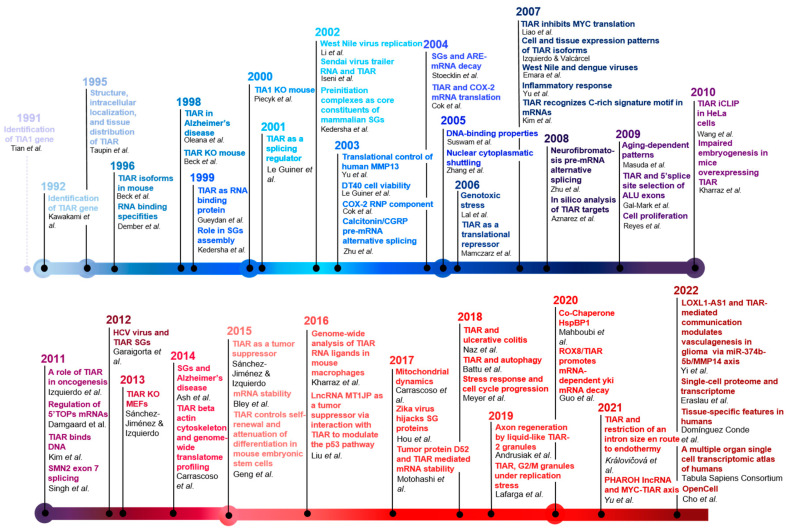
Since then, much effort has been directed towards studying and characterizing this gene and its products, including the different isoforms, its protein structure and organization, and its selective and specific interaction with RNA sequences. Likewise, its multiple functions in cellular processes and in pathology have been extensively investigated, as evidenced by the wealth of published information during the past three decades (Figure 1).
Figure 1.
Chronology of TIAR research publications. Bar graph-like representation of TIAR years/publications searching for TIAR/TIAL1 terms in PubMed (https://pubmed.ncbi.nlm.nih.gov; accessed on 13 June 2022).
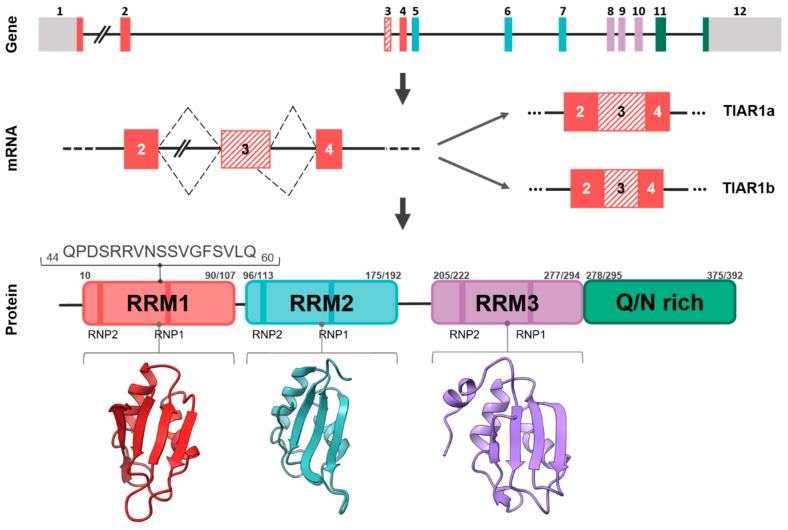
The human TIAR gene consists of 12 exons located on the chromosomal region 10q [3] (Figure 2). The correspondence between exons and the functional domains of the protein are as follows: exons 1–4 code for RNA-recognition motif/module 1 (RRM1), exons 5–7 for RRM2 and exons 8–10 for RRM3. Exon 11 and the most 5′ part of exon 12 encode the disorganized prion-like domain corresponding to the carboxyl terminus (Figure 2) together with the 3′-untranslated region (3′-UTR) of its mature mRNA. Two major TIAR isoforms have been identified in vertebrates—TIARa (50 kDa) and TIARb (42 kDa)—which differ in 17 amino-acid peptides located between the ribonucleoprotein (RNP)1 and RNP2 motifs of RRM1. This additional peptide sequence in TIARa is the result of an alternative-splicing event in the last 51 nucleotides of exon 3 [3] (Figure 2). This extra peptide confers specificity to TIARa for the recognition of specific RNA sequences, as well as the potential for interaction with other proteins and/or post-translational modifications [4]. The exonic and intronic organization TIAR is conserved between mouse and human species and it is located in the 7F4 region of the murine genome [3]. Nowadays, there is little scientific evidence about the differential regulatory role between main TIAR isoforms (a and b); therefore, it will be an interesting challenge to be studied because characteristic patterns of TIAR isoforms could determine specific cellular interactome among proteins and/or RNAs with potential impacts on gene expression flux, biological processes and their pathophysiological consequences.
Figure 2.
Illustrative representation of human TIAR isoforms and gene. Collection of exons and introns of the main isoforms, a and b, generated by alternative splicing, as well as functional domains. TIAR contains three RNA-recognition motifs (RRMs) and an auxiliary domain IDR rich in asparagine and glutamine (Q/N-rich domain). The location and position of amino acid sequences on RRM1 that differentiate the a and b isoforms is shown above a spacer. The secondary–tertiary structures of each of the three RRMs are shown.
TIAR is a modular RBP composed of three characteristic RRMs, highly conserved with those found in TIA1 [3,4,5,6], and an unstructured or disorganized domain (IDR) in the carboxyl-terminal region (Figure 3) [7]. Its C-terminal domain also possesses a lysosome-targeting motif [1,7]. The major TIARa and TIARb isoforms are composed of 392 and 375 amino acid residues, respectively.
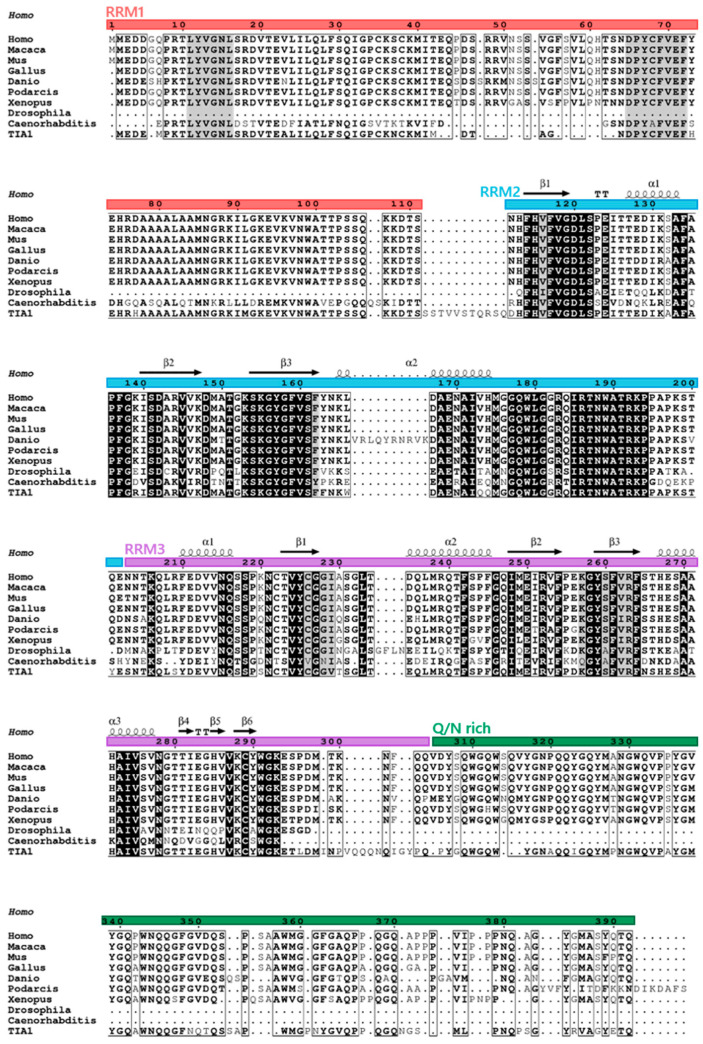
Figure 3.
High degree of conservation of TIAR orthologs. Alignments of TIARs from different species as well as human TIA1 are shown. Primary sequences of amino acids used in the analysis are as follows: Homo sapiens (Human) TIAR, PI_AAA36384.1; Macaca mulatta (Rhesus monkey) TIAR, XP_015003791.1; Mus musculus (Mouse) TIAR, PI_AAC52870.1; Gallus gallus (Rooster) TIAR, PI_AAO49721.1; Danio rerio (Zebrafish) TIAR, NP_957426.1; Podarcis muralis (Lizard) TIAR, XP_028585916; Xenopus tropicalis (Western clawed frog) TIAR, NP_001356541.1/NM_001369612.1; Drosophila melanogaster (Fruit fly), NP_001303550.1; Caenorhabditis elegans (Nematode), NM_064317.2; Homo sapiens (Human) TIA1, NP_071505.2.
At the amino acid level, TIAR and TIA1 share 85% homology in the amino-terminal region; specifically, RRM1–3 share 79%, 92% and 91% homology, respectively, and the C-terminal region shares 51% homology (Figure 3). As well as having structural similarities, TIAR, as a TIA1 paralog and vice versa, has overlapping functions with TIA1 in the field of gene expression, cellular events and pathophysiology, for instance, because they regulate specific and overlapping aspects of the transcriptome, translatome and interactome (RNA and/or protein complexes), suggesting that their functional effects can be redundant, additive and even independent [8], as we will see below.
2. Phylogenetics and Cellular/Tissular Expression Profiling
Structural orthologs of TIAR exist in different taxonomic groups, likely because of the evolutionary role of RNA and RBPs, and their functional role in modulating and adapting the RNA world to that of DNA and proteins. This implies the existence of a common ancestor that has been shaped throughout evolution, and the segregation and specialization of the multifunctional activities of the many RBPs with additional functions acquired during the modular assembly. The structural and functional orthologs of TIAR proteins in vertebrate and non-vertebrate taxonomic groups are shown in Figure 3.
The RRM domains have been characterized in detail, and much information is available on their topology and structure [9,10,11]. Similar to TIA1, the RRM2 of TIAR is the main RNA and DNA sequence-specific interaction domain, showing preferences for uracil- and/or adenine-, and cytidine-rich sequence repeats, termed ARE (AU-rich element) and CU-rich sequences [4,12,13]. This sequence-dependent specificity is further extended to cytosine- and uracil-rich sequences by the participation of RRM3 [13]. RRM1–3 of TIAR have been crystallized and are under intense investigation by different laboratories [reviewed in 5]. However, as in the case of TIA1, precise information on the intimate structural details as well as methodology to address the experimental challenges represented by the structural “Pandora’s box” located in the carboxyl-terminal disorganized region (IDR) is lacking. Likely, the development of new methodologies and algorithms will help to unravel its grammar and language within the proteomic universe of networks, and elucidate its functional dynamics [8].
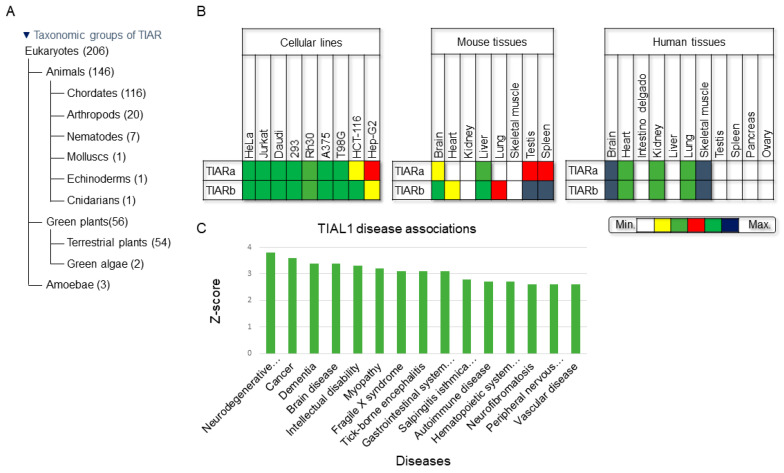
TIAR is ubiquitously expressed in several cellular types in all tissues within the eukaryotic kingdom [14] (Figure 4A). TIAR exhibits a low cell-type specificity in vertebrate/human cells and tissues (Figure 4B), as reported in the human protein atlas [15]. Similarly, single-cell profiling involving massive analysis of proteome and transcriptome data, including from associated diseases, revealed low cell immune specificity and low human brain regional specificity [16,17,18]. However, it is known that there is regulatory crosstalk among many major RBPs––for example, several findings underscore the notion that the expression, turnover and translation of regulatory RBPs (including AUF1, HuR, KSRP, NF90, TIA1 and TIAR) are controlled, at least in part, at the post-transcriptional level through a complex circuitry of self- and cross-regulatory RNP interactions as well as through the tissue- and age-dependent expression of RBPs that influence mRNA turnover and translation [19,20] (Figure 4B).
Figure 4.
Phylogenetic graphics, cellular- and tissular-expression patterns and TIAR-associated diseases based on the Jensen Laboratory list. (A) Taxonomic groups of TIAR according to the National Center for Biotechnology Information database. (B) Expression profiling of TIAR in human cell lines and in human and mouse tissues. HeLa (uterine carcinoma), Jurkat (T lymphoma), Daudi (B lymphoma), HEK293 (human embryonic kidney), Rh30 (bone marrow rhabdomyosarcoma), A375 (melanoma), T98G (glioblastoma), HCT-116 (colon carcinoma) and Hep-G2 (liver carcinoma). (C) Gene ontology of human diseases associated with TIAL1/TIAR (identifier: (ENSP00000358089)) expression, according to Jensen’s list, derived from automatic text mining of the biomedical literature, database annotations, cancer mutation data and genome-wide association studies (https://diseases.jensenlab.org/Entity?order=textmining,knowledge,experiments&textmining=10&knowledge=10&experiments=10&type1=9606&type2=-26&id1=ENSP00000358089, last access on 25 June 2022).
From a clinical perspective, several diseases/disorders are linked to TIAR expression/dysfunction, including tumorigenesis, acute inflammatory responses, autoimmunity, infectious diseases and neurological disorders. Some of these diseases are summarized in the list compiled by the Jensen laboratory and classified by Z-score of TIAR/TIAL1 disease associations (Figure 4C). Further, TIAR is an abundant protein in many eukaryotic cells. For example, a recent study has estimated its concentration in HEK-293T cells at around 1100 nM and 6.9 × 105 copies/cell compared to that of TIA1, at 630 nM and 3.8 × 105 copies/cell [21].
2.1. Gene Expression Control
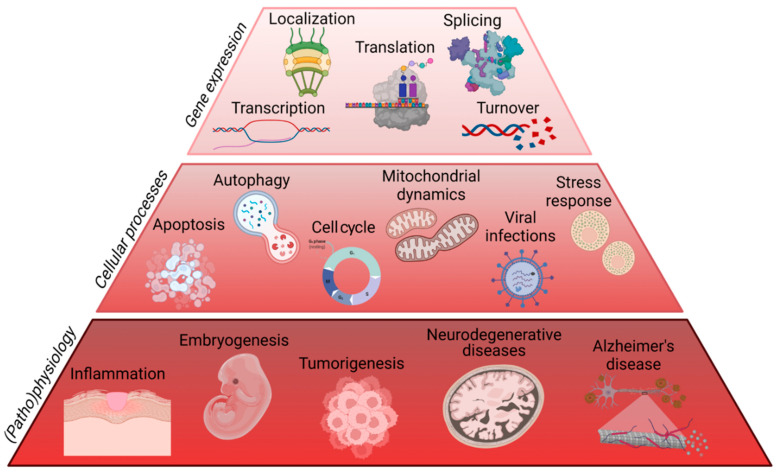
As a DNA- and RNA-binding protein, TIAR is involved in myriad processes related to gene expression regulatory flux, including DNA replication/transcription, processing and splicing of pre-mRNAs, location, stability and translation of mature mRNAs, as well as post-translational regulatory events (Figure 5 and Figure 6).
Figure 5.
The multifunctional characteristics of TIAR on gene expression flux, cellular events and pathophysiological situations. Figure created with BioRender.com (accessed on 12 June 2022).
Figure 6.
Breakthroughs of the TIAR timeline. To build this figure, we utilized the following references: [1,2,3,4,7,12,13,14,16,17,18,21,23,24,26,29,32,33,34,35,37,38,39,41,42,44,45,46,47,48,49,54,55,56,57,61,62,67,68,69,71,72,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106].
2.2. Transcription
TIAR can modify the transcriptional rates of RNA polymerase II through interactions with components of the transcriptional machinery [22] and its affinity for single- and double-stranded DNA [23,24]. The depletion of TIAR in F9 cells is a good functional example of this interaction, as it affects the promoter activity of an 80 bp fragment of the Pituitary adenylate cyclase-activating polypeptide (PACAP) gene, suggesting that it might be involved in testis-specific gene transcriptional regulation. PACAP is a pleiotropic neuropeptide localized in the testis at concentrations comparable with those found in the brain, indicating that it is involved in spermatogenesis [25]. Similarly, the genome-wide analysis of the transcriptome of TIAR-depleted HeLa cells identified a large number of partner mRNAs associated with inflammation, cellular signaling, immune response, angiogenesis, apoptosis, metabolism and cell proliferation [26].
2.3. Alternative Splicing
It was demonstrated early that TIAR interacts with selective and specific RNA motifs [4,12,13]. To participate in the control of alternative pre-mRNA splicing [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43], TIAR binds to uridine-rich sequences, which are mostly located in the introns, and seems to facilitate the recruitment of the U1 small nuclear ribonucleoprotein, thus promoting the recognition and processing of atypical 5′ splice sites [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43].
TIAR was also identified as a novel player in the regulation of human calcitonin/CGRP alternative RNA processing [42]. TIAR bound to the U-tract sequence motif downstream of a pseudo 5′ splice site within a previously characterized intron enhancer element. The binding of TIAR promoted the inclusion of the alternative 3′-terminal exon located more than 200 nucleotides upstream from the U-tract. In cells that preferentially include this exon, the overexpression of a mutant TIAR lacking the RNA-binding domains suppressed the inclusion of this exon. In this cellular context, an unusual novel interaction was demonstrated between U6 small nuclear (sn)RNA and the pseudo 5′ splice site, which was shown previously to bind U1 snRNA. Interestingly, TIAR binding to the U-tract sequence depends on the interaction of not only U1 but also U6 snRNA with the pseudo 5′ splice site. Conversely, TIAR binding promotes U6 snRNA binding to its target. The synergistic relationship between TIAR and U6 snRNA strongly suggests a novel role for U6 snRNP in regulated alternative RNA processing [42]. TIAR has also been associated with tissue-specific splicing events [43].
TIAR can be displaced between the nucleus and cytoplasm in a specific sequence-dependent manner, as mutations of the highly conserved RNP2 or RNP1 peptides in RRM2 redistribute TIAR to the cytoplasm, and similar modifications in RRM3 abolish TIAR nuclear exports [44].
2.4. Translation
The post-transcriptional control of mRNA metabolism is mediated by RBPs, together with long non-coding RNAs (lncRNAs) and/or microRNAs (miRNAs), which are assembled with cellular transcripts forming transient and dynamic RNP particles that define the life and fate of cellular mRNAs in the short and medium term (in the absence of transcriptional activity) in environmental-dependent contexts. The three RRMs of TIAR allow its interaction with specific sequences localized in the UTRs of ~5% of the human transcriptome [12,13,33,35,38,39]. Consequently, TIAR might be considered as a master regulator of the translation of many cellular mRNAs [12,13,33,35,38,39]. This control is selectively exerted through the recognition of AU- and CU-rich sequences located on 5′- and 3′-UTRs on cellular mRNAs [12,13,33,35,38,39,45,46]. Some of most representative mRNAs, whose translation is regulated/mediated by TIAR, include the following: human matrix metalloproteinase-13 (MMP13) in a TIARa isoform-dependent manner [47], cyclooxygenase-2 (COX-2/PTGES) [48,49], β2-adrenergic receptor [50,51,52], Xenopus laevis Vg1 [53], GADD45alpha [54], cytokines [55], c-myc [56], 5′-terminal oligopyrimidine tract mRNAs [57,58], insulin [59], alpha-synuclein [60] and potential components of the cellular and translational machinery [61,62]. TIAR is also associated with translational repressor structures that form cytoplasmic foci similar to stress granules (SGs) [63]. Another translational repressor mechanism involves the interaction of TIAR with canonical components of the cellular translational machinery, such as eIF4GI, in acute myocardial ischemia [64].
RBPs are subject to post-translational modifications (PTMs) that continuously adjust their activity to maintain cell homeostasis. PTMs can dramatically change the subcellular localization, the binding affinity for RNA and protein partners, and the turnover rate of RBPs. Moreover, the ability of many RBPs to undergo phase transition and/or their recruitment to previously formed membrane-less organelles, such as SGs, is also regulated by specific PTMs [65,66].
2.5. Turnover/Stability
ARE sequences have been shown to be binding sites for numerous RBPs including TIAR, revealing a role in the coordination/control of gene expression through the regulation/modulation of stability and turnover of cellular RNAs [67,68,69]. Several examples of human mRNAs regulated by TIAR at this level have been identified through the use of loss- and gain-of-function cell models testing both endogenous and chimeric mRNAs. Some relevant examples include iNOS [70], TPD52 [71] and alpha-synuclein [60] mRNAs. Stability/turnover events can involve RNAs and SGs [72], and also non-coding RNAs (lncRNAs/miRNAs) [73,74,75,76,77,78,79,80]. An example of this is the Hippo pathway, which is a regulator of organ growth and tumorigenesis. In Drosophila, oncogenic RasV12 cooperates with loss-of-cell polarity to promote Hippo-pathway-dependent tumor growth. Mechanistically, Rox8 (the Drosophila ortholog of TIAR) directly binds to a target site located in the yki 3′ UTR and recruits and stabilizes the targeting of miR-8-loaded RNA-induced silencing complex, which accelerates the decay of yki mRNA [73].
2.6. lncRNA- and miRNA-Mediated Regulation
As mentioned above, several molecular, functional and regulatory links have been identified between TIAR and distinct classes of cellular ncRNAs. For example, the lncRNA MT1JP functions as a tumor suppressor by interacting with TIAR to modulate p53 signaling [74]. Similarly, the lncRNA PHAROH regulates Myc translation in hepatocellular carcinoma by sequestering TIAL1 [75], and the lncRNA LOXL1-AS1 interacts with TIAR to modulate vasculogenic mimicry in glioma through the regulation of the miR-374b-5p/MMP14 axis [76]. Interestingly, several single nucleotide polymorphisms in lncRNA regions found in TIAR are associated with breast cancer risk [77]. The participation of miRNAs has also been reported—for example, the interaction between miR-223-3p and TIAL1, which downregulates TIAL1, is involved in the neuroprotective effects of dexmedetomidine on hippocampal neuronal cells in vitro [78], as well as vasculogenic mimicry in glioma via the regulation of the miR-374b-5p/MMP14 axis [76]. As stated earlier, TIAR/Rox8 promotes miRNA-dependent yki messenger RNA decay [73]. TIAR coupled with miR-579 and miR-125b participates in combined transcription- and translation-repressive events to tightly regulate pro-inflammatory gene expression in leukemic THP-1 cells during endotoxin tolerance, a common feature of severe systemic inflammation [79].
TIAR also appears to be involved in the extracellular trafficking of cell-derived microvesicles, which is a novel mechanism of cell-to-cell communication. The finding that microvesicles contain RNPs involved in the intracellular traffic of RNA and selected miRNAs suggests a dynamic regulation of RNA compartmentalization with potential biological effects [80].
2.7. Tissular and Cellular Homeostasis
Tissular homeostasis can be perturbed by diverse cellular events, and the associated adaptations in cellular function require the participation of RBPs, including TIAR, to drive transcriptional/post-transcriptional changes to improve health cellular dynamics features versus degenerative phenotypes in human diseases/disorders.
2.8. Autophagy
Autophagy is a natural catabolic process of regeneration in which intracellular material is degraded in vesicular structures called autophagolysosomes. Autophagy is necessary for normal cell and tissue function, and to stop the accumulation of misfolded, damaged and aggregated proteins and other toxic substances. Impaired autophagy is associated with various human disorders, especially neurodegenerative diseases. Ablation of TIAR in mouse embryonic fibroblasts stimulates high rates of adaptive autophagy [93]. Contrastingly, transcriptome-wide analysis links the short-term expression of TIARb to a protective proteostasis-mediated cell quiescence response [95,107].
The activation of the amino acid starvation response (AAR) is known to increase lifespan and acute stress resistance, and to also regulate inflammation. The pharmacologic activation of AAR by halofuginone was shown to significantly inhibit the production of the proinflammatory cytokine interleukin 1β (IL-1β) and provide protection from intestinal inflammation in mice. Halofuginone inhibits IL-1β through general control nonderepressible 2 kinase (GCN2)-dependent activation of the cytoprotective integrated stress response pathway, resulting in the rerouting of IL-1β mRNA from translationally active polysomes to inactive ribocluster complexes—such as SGs—via the recruitment of the TIA1 and TIAR, which are further cleared through the induction of autophagy [101]. Likewise, translationally stalled IL-1β mRNAs recruit TIA1 and TIAR, resulting in RBP-RNA SG complexes. The SG pathway regulates IL-1β production, and bound IL-1β mRNAs might undergo degradation through the induction of autophagy [108]. TIAR is also a component of the RNP in the control of endotoxin-induced macrophage responses [109].
2.9. Apoptosis
Apoptosis is an essential process of tissular homeostasis to remove damaged and unneeded cells. Inappropriate apoptosis (either too little or too much) is a major factor in many human diseases, including neurodegenerative diseases, ischemic damage, autoimmune disorders and many types of cancer. The ability to modulate the life or death of a cell has immense therapeutic potential. One of the first reports on TIAR described its involvement in stress-induced apoptosis [7], as it triggers DNA fragmentation in permeabilized thymocytes, and can redistribute from the cell nucleus to the cytoplasm during Fas-induced cell death. Similarly, TIAR gain-of-function models in HEK293 cells trigger an apoptotic phenotype in a p53 pathway-associated cellular response [95]. In the same vein, the C. elegans TIA1/TIAR homolog, TIAR-1/TIAR-2, is required for germ cell apoptosis [105,110], and a correlation exists between thyroid disease and excessive apoptosis in thyroid tissues associated with elevated TIAR expression [111]. Regardless, there is a commonality between TIA1 and TIAR since their complete inactivation in HEK293 cells leads to mitotic catastrophe and cell death [38].
2.10. Cell Cycle
Primordial germ cells (PGCs) give rise to both eggs and sperm via complex maturational processes that require both cell migration and proliferation. TIAR is essential for PGC development [81]. In response to DNA damage, the p38/MK2 complex is relocated from the nucleus to the cytoplasm where MK2 phosphorylates hnRNPA0 to stabilize GADD45alpha mRNA, while p38 phosphorylates and releases TIAR, suggesting a role for the MK2 pathway in the post-transcriptional regulation of gene expression as part of the DNA damage response in cancer cells [65].
TIAR is also involved in the timeline of cell proliferation through the cell cycle [26,38,91,93,104]. Indeed, TIAR accumulates in nuclear G2/M transition granules (GMGs) induced by replication stress. During G2/M checkpoint activation, TIAR retains CDK1 in GMGs, attenuates CDK1 activity and, thereby, promotes genome stability [104]. TIAR controls mitotic entry and is required for G2/M checkpoint activation independently of the ATR-Chk1 pathway. During G2 and prophase, TIAR accumulates in GMGs, which contain factors involved in transcription, splicing, DNA replication, DNA repair, as well as CDK1/Cyclin B, and may be sites of stalled replication. In addition, loss- and gain-of-function cell models of TIAR have highlighted their participation in the modulation of the proliferation of several transformed cell lines [26,38,56,57,58,91,99,100,104,112,113].
2.11. Mitochondrial Function
Mitochondrial biogenesis is a complex cellular process involving two separated and compartmentalized genomes and several regulatory players that function as transcription factors and/or RBPs to coordinate both genetic systems [114]. Experimental evidence from loss- and gain-of-function TIA cell models has revealed modifications of mitochondrial phenotypes, affecting mitochondrial function and morphology/architecture [93,99,100,115]. TIAR has been implicated in regulatory events associated with the post-transcriptional control of splicing, translation and/or stability of nuclear-encoded mitochondrial mRNAs, and modulates the splicing and translation/stability of human OPA1 mRNA [100]. In fact, TIAR can potentially bind and target about 345 and 678 human mitochondrial mRNAs as revealed by HeLa TIAL1 iCLIP [35] and HEK293 PARCLIP [38] analysis, respectively. Of them, 288 targeted mRNAs are potentially shared between the two experimental approaches, strongly supporting their modulation by TIAR. These nuclear-encoded mitochondrial components are associated with mitochondrial organization, metabolic processes, respiration, generation of precursor metabolites and energy, and respiratory electron transport chain activity [35,38]. Many of the potential targets can also be sequestered—for example, during stress responses, such as oxidative stress or damaged DNA response. TIAR can also modulate mitochondrial activity through mitochondrial master regulators, such as NFE2L2/Nrf2, a TIAR-targeted mRNA [116]. The available experimental evidence clearly indicates that Nrf2 is an important player in the maintenance of mitochondrial homeostasis and structural integrity. Its role is particularly critical under conditions of oxidative, electrophilic or inflammatory stress, when the ability to upregulate Nrf2-mediated cytoprotective responses influences the overall health and survival of the cell. As many human pathological conditions involve oxidative stress, inflammation and mitochondrial dysfunction, the pharmacological activation of Nrf2 holds promise for disease prevention and treatment [117].
2.12. Cellular Stress
In response to stressful conditions, eukaryotic cells launch an arsenal of regulatory programs to protect the proteome and the transcriptome. One major protective response involves the arrest of protein translation and the formation of SGs, which we have mentioned are discrete cytoplasmic inclusions containing non-membranous RNP complexes with abundant RBPs such as TIAR. The SG response is thought to ensure survival and to preserve cell viability when conditions improve. Similar to TIA1, TIAR is a central player in SG formation, structure and function [83,84].
The three RRMs of TIAR enable the selective binding to RNA, and its prion-like domain allows TIAR to reversibly aggregate to form SGs [3,4,5,6]. The overexpression of the TIAR prion domain is sufficient to induce SG formation [83]. Distinct macromolecular interactions lead to the phase separation of protein and RNA during stress, such as protein–protein, RNA–protein and RNA–RNA interactions. While the identities of many proteins and RNAs contained in SGs have been recently elucidated using different experimental approaches [118,119], the function of this conserved compartmentalization of the cytoplasm during stress response remains elusive.
That being said, accumulating evidence points to an antiviral nature of SGs, which is supported by the discovery of many viral factors that interfere with SG formation and/or function [72,120,121]. SGs are, however, dispensable for mRNA stabilization during cellular stress [96]. For example, in C. elegans, salt stress, oxidative stress and starvation, but not heat shock, induce the relocalization of ubiquitin, proteasome and TIAR-2 into distinct subnuclear regions referred to as stress-induced nuclear granules (SINGs) [122]. In the case of viral infections (Sendai virus), virus-expressed factors enabling a well-balanced ratio of suppression and triggering of apoptosis are thought to be essential for optimal virus replication [123].
Results from the depletion of G3BP1 and TIA1/TIAR in senescent cells revealed that loss of G3BP1 contributes to impaired SG formation. Aging reduces Sp1 levels, and this transcription factor regulates G3BP1 and TIA1/TIAR abundance, suggesting that the decline in SG formation can provide a new biomarker to evaluate cellular aging [124]. Further, the migration of the splicing factor ASF/SF2 into SGs is strictly determined both by its shuttling properties and its RNA-binding capacity, and it cooperates with TIA proteins in the regulation of mRNA metabolism under normal conditions and also under conditions of environmental stress [125]. For example, TIAR is one of the mRNA processing factors involved in mammalian hibernation [126].
Human astrocytoma cell SGs contain mRNAs that are known to be involved in glioma signaling and the mammalian target of rapamycin pathway, involving proteins such as the cytokinetic proteins epithelial cell transforming 2 and Aurora kinase B (AURKB) together with canonical components of SGs, such as TIAR and G3BP1 [127]. TIAR is also a component of SGs in pluripotent stem cells under stress conditions, such as oxidative stress and heat shock [128].
A recent study revealed that RNA granule components including 2 key SG RBPs with low-complexity prion-like domains—PAB-1 and TIAR-2—aggregate in aged C. elegans in the absence of disease [129]. This study presented new evidence that sustained SG formation triggers RBP aggregation. In addition, the authors demonstrated that mild chronic stress during aging promotes mislocalization of nuclear RBPs. These findings shed light on how age-related changes can contribute to pathogenesis in neurodegenerative diseases and the disruption of RNA homeostasis [129,130].
A higher number of cells with granules, which persist for longer periods than in controls and ALS cases, represents an early molecular change occurring before ALS onset, suggesting a transient pre-aggregative state [131]. In the case of HspBP1, it is associated with the SG proteins G3BP1, HuR and TIA1/TIAR. HspBP1 also interacts with poly(A)-RNA in vivo and binds directly RNA homopolymers in vitro. Multiple lines of evidence and single-granule analyses demonstrate that HspBP1 is crucial for SG biogenesis [106].
Interestingly, Drosophila orthologs of the mammalian SG components AGO1, ATX2, CAPRIN, eIF4E, FMRP, G3BP, LIN-28, PABP and TIAR are enriched in adult fly intestinal progenitor cells, where they accumulate in small cytoplasmic messenger RNP complexes (mRNPs). Treatment with sodium arsenite or rapamycin was shown to reorganize these mRNPs into large cytoplasmic granules or intestinal progenitor stress granules (IPSGs), and this depended on polysome disassembly, which resulted in translational downregulation, and was reversible. Although the canonical SG nucleators ATX2 and G3BP were sufficient for IPSG formation in the absence of stress, neither of them, nor TIAR, either individually or collectively, were required for stress-induced IPSG formation [132].
The pathophysiological importance of SGs and their RNP components in the formation, progression and metastatic fate of several human solid tumors have been recently reported and reviewed [133,134]. It is known that genome integrity must be tightly preserved to ensure cellular survival and to deter the genesis of disease. Endogenous and exogenous stressors that impose threats to genomic stability through DNA damage are counteracted by a tightly regulated DNA-damage response. TIA proteins and other RBPs are emerging as regulators and mediators of diverse biological processes to maintain genome integrity and prevent deleterious phenotypes in cellular scenario/conditions associated with genotoxic stress [135].
2.13. Viral Biology
In the course of viral infectious cycles, many nuclear–cytoplasmic shuttling proteins of mostly nuclear distribution are retained in the cytoplasm by viruses and re-purposed. Indeed, several mammalian viruses hijack a common group of factors—for example, cytoplasmic RNA viruses use host nuclear factors in new functional roles supporting virus translation and virus RNA replication, a common theme employed by different virus groups [136]. TIAR is one of these co-opted proteins in SG assembly in response to environmental stress, including viral infections.
As mentioned earlier, there is accumulating evidence for the antiviral nature of SGs. Indeed, viruses have evolved diverse mechanisms to prevent the formation of SGs and enable the synthesis of viral proteins using host translation machinery. SGs facilitate the establishment of an antiviral state by limiting viral protein accumulation and regulating signaling cascades that affect virus replication and immune responses. Mechanisms have been described that allow the ongoing translation of mRNPs that encode antiviral factors, such as interferon-stimulated genes (ISGs), despite the arrest of bulk translation [137,138,139].
TIAR (and also TIA1) translocate from the nucleus to the cytoplasm after EV71 infection and localize to sites of viral replication. TIA proteins can facilitate EV71 replication by enhancing viral genome synthesis in host cells. Both proteins were reported to bind the stem-loop I of the 5′-UTR of the EV71 genome and improve the stability of viral genomic RNA [140]. A role for TIAR in facilitating viral replication has also been associated with the West Nile virus [86,89]. TIAR is also removed/sequestered by the Sendai virus [85], and by the West Nile and dengue viruses [88].
By contrast, the formation of SGs is induced early during poliovirus infection [141], but this ability is lost as the infection proceeds, and SGs disperse. Infection resulted in the cleavage of G3BP, but not of TIA1 or TIAR, by the poliovirus 3C proteinase. The expression of a cleavage-resistant G3BP mutant restored SG formation during poliovirus infection and significantly inhibited virus replication. These results elucidate a mechanism for viral interference with mRNP metabolism and gene regulation, and support a differential critical role of RBPs in SG formation and the restriction of virus replication [141].
During HIV-2 infection, TIAR associates with genomic RNA to form a TIAR-HIV-2 ribonucleoprotein complex diffusely localizing in the cytoplasm or aggregated in SGs [142]. Moreover, the HIV-1 Gag protein blocks SG assembly irrespective of eIF2α phosphorylation, and even when SG assembly is forced by the overexpression of G3BP1 or TIAR [143].
Another example is represented by the porcine reproductive and respiratory syndrome virus (PRRSV), which induces SG formation via a PERK-dependent pathway in MARC-145 cells, with SGs involved in the signaling pathway of the PRRSV-induced inflammatory response [144].
Previous studies reported TIA1/TIAR recruitment at sites of flavivirus replication, and recent work has expanded on these observations and demonstrates that, similar to TIA1, TIAR behaves as an inhibitor of viral replication [145]. The approach used by the authors, using RNA interference in human cells, contradicts the previous hypothesis based on mouse embryonic fibroblast knockout studies, and shows that tick-borne encephalitis virus (TBEV) is capable of inducing bona fide G3BP1/eIF3/eIF4B-positive SGs together with a differential phenotype of stress response proteins following viral infection, implicating TIA1 in viral translation and as a modulator of TBEV replication [145].
Some, but not all, flavivirus-capsid proteins also block SG assembly, suggesting differential interactions between flaviviruses and SG biogenesis pathways. The depletion of the SG components G3BP1, TIAR, and Caprin-1, but not TIA1, reduced Zika virus (ZIKV) replication [146]. These results are consistent with a scenario in which ZIKV uses multiple viral components to hijack key SG proteins to benefit viral replication [101]. However, the knockdown of TIA1 and TIAR affected ZIKV protein and RNA levels but not viral titers. Conversely, the depletion of Ataxin2 and YB1 decreased virion production, despite having only a small effect on ZIKV protein expression. This study provides new insights into virus–host interactions and identifies the potential contribution of TIA proteins to the unusual pathogenesis associated with this reemerging arbovirus [146].
However, human papillomaviruses sequester TIAR to repress the formation of SGs [147]. By contrast, hepatitis C virus (HCV) induces the formation of SGs, whose proteins regulate HCV RNA replication and virus assembly and egress [92] and the same occurs with vesicular stomatitis virus [148]. Porcine reproductive and respiratory syndrome virus (PRRSV)-induced SGs are associated with viral replication complexes, and also TIAR, and suppression of host translation [149].
Poliovirus 2A protease triggers a selective nucleo-cytoplasmic redistribution of splicing factors (including TIA proteins) to regulate alternative pre-mRNA splicing [150]. TIAR has been linked to the binding downstream of the nonconsensus donor of the large intron present in the nonstructural gene of minute virus of mice to regulate of splicing [151].
3. Physiology and Pathology
3.1. Inflammation
The functional roles of RBPs in immunity and its associated diseases are well known. The dysregulation of RBPs and their targets result in chronic inflammation and autoimmunity [26,46,55,152,153]. TIAR is a well-known attenuator of inflammation and, accordingly, it has been extensively studied as a key post-transcriptional regulator of inflammation and immune response. TIAR can collaboratively or competitively bind the same target mRNAs with other RBPs, such as AUF1, ELAVL1/HuR, KSRP, TIA1, TTP, Roquin or Regnase, to enhance or dampen regulatory activities [152,153]. These RBPs can also bind their own 3′-UTRs to negatively or positively regulate their expression. Both upstream signaling pathways and miRNA regulation shape the interactions between RBPs and target RNAs. In myeloid cells, TIAR has been shown to bind and regulate the translation and stability of various mRNA-encoding proteins important for inflammatory response, such as TNFα [45,55], Cox-2 [48,49], many proinflammatory cytokines [55], and IL-8 [154]. A study in macrophages using a combination of RNA-IP and microarray analysis (RIP-chip) identified over 400 mRNAs specifically bound by the full-length protein in response to endotoxin [98].
The dysregulation of RBPs results in chronic inflammation and autoimmunity. In this regard, a transcriptome meta-analysis identified an immune signature involving RBPs in the immune cells of patients with ulcerative colitis (UC), who showed significantly lower TIAR expression compared with healthy controls [103]. In the same study, the deletion of TIAR in macrophages using siRNAs resulted in an enhanced production of the inflammatory cytokine IL-1β [103]. By contrast the aberrant expression of TIA1 and TIAR has been documented in patients with rheumatic diseases, leading to the production of autoantibodies to TIA proteins, specifically, an increased prevalence in systemic lupus erythematosus and systemic sclerosis and correlations with clinical features [155].
Another noteworthy aspect is neutrophilic inflammation in asthma, which is associated with interleukin (IL)-17A, corticosteroid insensitivity and bronchodilator-induced forced expiratory volume in 1 s (FEV1) reversibility. IL-17A synergizes with TNF-α in the production of the neutrophil chemokine CXCL-8 by primary bronchial epithelial cells (PBECs). At the molecular level, epithelial hyper-responsiveness was associated with the failure of TIAR to translocate to the cytoplasm to halt CXCL-8 production, as confirmed by TIAR knockdown [156]. This is in line with the finding that hyper-responsive PBECs also produce enhanced levels of other inflammatory mediators. Normalizing the cytoplasmic translocation of TIAR is thus a potential therapeutic target in neutrophilic, corticosteroid-insensitive asthma [156].
TIAR has also been identified as a potential component of a gene signature linked to pulmonary sarcoidosis, as it was downregulated in patients with sarcoidosis compared with healthy individuals [157]. Nevertheless, further studies are required to evaluate the precise role for TIAR in inflammatory scenarios linked to human pathologies, such as autoimmunity, arthritis, ulcerative colitis, ulcerative colitis, asthma or pulmonary sarcoidosis.
3.2. Embryogenesis
The phenotypic differences observed between mice with the inactivation of TIAR [81] and/or TIA1 [46] indicate that they may cooperate or act independently during early embryogenesis. The phenotype of mice lacking TIAR appears to depend on the mouse strain in which the studies are performed. TIAR deficiency resulted in embryonic lethality in 100% of BALB/c mice, but only in 90% of C57BL/6 embryos. Crossing BALB/c TIAR +/− mice with C57BL/6 TIAR +/− mice produced 60% embryonic mortality. Of the remainder, half of the mice survived to adulthood but were sterile with abnormalities in spermatogenesis and oogenesis, as well as in the architecture of the gonads themselves. Other phenotypes included obesity, despite being born with lower body mass, and neurological disorders. In addition, the mice developed cervical cancer as adults [81]. Conversely, in a transgenic mouse model of TIAR overexpression, 77% of embryos had abnormalities at embryonic day 7.5 [90]. TIAR was also reported to control self-renewal and the attenuation of differentiation in mouse embryonic stem cells [97]. Overall, embryonic and germ cell development, as well as the differentiation of murine embryonic stem cells, are compromised by the reduction/absence or overexpression of TIAR [46,81,90,93,97,158]. In the case of the C. elegans, the TIA-1/TIAR homolog TIAR-1/TIAR-2 is required to induce germ cell apoptosis, and TIAR-1 protects female germ cells from heat shock [105,110,159].
3.3. Carcinogenesis
TIAR/TIAL1 has been studied in several transformed cells and solid tumors. The first seminal work showed that TIAR regulates translation of the c-myc oncogene mRNA in a 3′-UTR in K652 cells [56]. In the same vein, the knockdown of TIAR expression in HeLa improves cell proliferation [26,91]. By contrast, using a cellular gain-of-function model in HEK293 cells, TIAR overexpression was shown to inhibit cell proliferation and trigger apoptosis and autophagy/mitophagy, indicating that TIAR functions as a tumor suppressor in a p53-dependent manner [95]. TIAR was also identified as a transformation/tumor suppressor in lung cancer tumors in an shRNA library-based genome-wide loss-of-function screen [113]. These observations have been reinforced with recent findings revealing the role of TIAR as a tumor suppressor via interaction with the lncRNA MT1JP to modulate the p53 pathway [74]. Furthermore, the downregulated expression of TIAR has been observed in several cell lines and tumor samples [91,95,112,113,160] and it is an unfavorable prognostic marker in liver cancer [15] and osteosarcoma [161].
The genome-wide analysis of transcript variation in breast cancer identified TIAR as involved in aberrant splicing. Patterns of transcript variant expression identified “hub” genes that differentiated the cancerous and normal transcriptomes, and the dysregulated expression of alternative transcripts may reveal novel biomarkers for tumor development [162].
The silencing of TIA proteins in several tumor cell lines triggers the upregulation of HIF-1α expression, and rapid and severe hypoxia causes co-aggregation of TIA proteins, which suppress HIF-1α expression, reflecting the control of HIF-1α expression by TIAR/TIA1 [163].
A very recent study has demonstrated a tumor suppressor role for TIAR in the incidence/progression of skin squamous cell carcinoma (SSCC) [160]. The downregulation of muscleblind-like protein 1 (MBNL1) promoted cell metastases (measured as Transwell migration) in SCL-1 cells, whereas the upregulation of MBNL1 reduced cell metastasis. Additionally, the downregulation of MBNL1 suppressed the protein expression of TIAR, myogenic determinant 1 (MyoD1) and caspase-3 in vitro. Consistent with these observations, the inhibition of TIAR or MYOD1 expression attenuated the effects of MBNL1 in SSCC. These observations reveal that MBNL1 suppresses the cancer metastatic capacity of SSCC via by TIAL1/MYOD1/caspase-3 signaling pathways [160].
TIAR is also a negative regulator of the BRCA1 oncogene; it has been shown to block translation and reduce the protein expression of BRCA1 in chronic myeloid leukemia cells, which leads to aneuploidy, spindle toxin resistance and genomic instability [164]. TIAR-mediated repression of BRCA1 mRNA translation is responsible for the downregulation of BRCA1 protein level in BCR-ABL1-positive leukemia cells. This mechanism may contribute to genomic instability [164]. Moreover, it is plausible that TIAR has the same effect on BRCA1 protein expression in breast cancer [77,164]. As already mentioned, TIAR interacts with LOXL1-AS1 to modulate vasculogenic mimicry in glioma via the miR-374b-5p/MMP14 axis. This observation might reveal novel targets for glioma therapy [76].
Nonetheless, an oncogenic or tumor suppressor function for TIAR (and their isoforms) could be highly dependent on the cell-type and the associated interactomes involving both RNA-protein and protein–protein interactions and dynamics [8,21]. TIAR ablation in murine embryonic fibroblasts compromises cell proliferation by delaying cell cycle at G2/M phase and triggering adaptive autophagy [93]. Additionally, the knockdown of TIAR accelerates mitotic entry and leads to chromosomal instability in response to replication stress, in a manner that can be alleviated by the concomitant depletion of Cdc25B or inhibition of CDK1. As TIAR retains CDK1 in GMGs and attenuates CDK1 activity, it was proposed that the assembly of GMGs may represent a hitherto unrecognized mechanism that contributes to the activation of the G2/M checkpoint [104]. The depletion of both TIA1 and TIAL1 paralogs by CRISPR-Cas9 technology drives cell death after 7 days [38].
Tumor protein D52 (TPD52) reportedly plays an important role in the proliferation and metastasis of various cancer cells, including oral squamous cell carcinoma (OSCC), and it is expressed strongly at the hypoxic center of the tumor and is involved in cell death resistance. This occurred through a mechanism involving enhanced mRNA stability by binding of the mRNA to TIA1 and TIAR [165]. The simultaneous knockdown of TPD52 and HIF-1α significantly reduced cell viability. In addition, in vivo tumor-xenograft experiments showed that TPD52 acts as an autophagy inhibitor caused by a decrease in p62. Thus, the expression of TPD52 increases in OSCC cells under hypoxia in a HIF-independent manner and plays an important role in the proliferation and survival of the cells in concordance with HIF [165].
Recently, it has been shown that glycolysis and tumor immunity are inter-related cellular events in osteosarcoma that share glycolysis-immune-related genes. TIAR is one of these genes and a potential candidate to construct a gene signature risk score to predict the prognosis of patients with osteosarcoma [164].
3.4. Neurodegenerative Diseases
The timing, dosage and location of gene expression flux are the main determinants of brain architectural complexity. In neurons, this is achieved by specific sets of RBPs and their associated factors, which bind to specific cis-elements throughout the RNA sequence to regulate splicing, polyadenylation, stability, transport and localized translation at both axons and dendrites. Not surprisingly, the misregulation of RBP expression or disruption of their function due to mutations or sequestration into nuclear or cytoplasmic inclusions have been linked to the pathogenesis of several neuropsychiatric and neurodegenerative disorders, such as fragile-X syndrome, autism spectrum disorders, spinal muscular atrophy, amyotrophic lateral sclerosis and frontotemporal dementia. The roles of TIAR and other RBPs have been analyzed by their specific molecular and cellular functions, the neurological symptoms associated with their perturbation and their axo-dendritic transport/localization along with their target mRNAs as part of larger macromolecular complexes termed RNP granules [166].
3.4.1. Neurofibromatosis Type I
Neurofibromatosis type I (NF1) is a common inherited autosomal-dominant disease that affects 1 in 3500 individuals with mutations that promote the loss of function of the NF1 protein, neurofibromin, which is involved in diverse signaling cascades. The disease is completely penetrant, but shows variable phenotypic expression in patients. NF1 is a large gene, and its pre-mRNA undergoes alternative splicing [27]. One of the best characterized occupations of NF1 is its function as a Ras-GAP (GTPase-activating protein). NF1 exon 23a is an alternative exon that lies within the GAP-related domain of neurofibromin. This exon is predominantly included in most tissues, and it is skipped in central nervous system neurons [27]. The isoform with the skipped exon 23a has 10-times greater Ras-GAP activity than the isoform, including exon 23a. This inclusion is tightly regulated by at least three different families of RBPs: CELF (CUG-BP, cytosine-uridine-guanine-binding protein) and ETR-3 (ELAV (embryonic lethal abnormal vision)-type RNA-binding protein-like factor) [167,168], Hu and TIA1 /TIAR. The CELF and Hu proteins promote exon 23a skipping, whereas the TIA1/TIAR proteins promote its inclusion [28,169]. The widespread clinical variability observed among patients cannot be explained by NF1 mutations alone and it is believed that modifier genes may have a role in the variability. The available information suggests that the regulation of alternative splicing may act as a modifier to contribute to the variable expression in NF1 [168].
3.4.2. Axon Regeneration
Axon regeneration is a coordinated and concerted process associated with various cellular events, including but not restricted to the injury sensing, axonal transport, synthesis of macromolecules, cellular energy homeostasis and cytoskeletal organization. Interestingly, a negative link between TIAR expression/post-translational modification and axon regeneration has been recently reported. Thus, C. elegans TIAR-2/TIAR protein functions cell autonomously to inhibit axon regeneration. TIAR-2 undergoes liquid–liquid phase separation in vitro and forms granules with liquid-like properties in vivo. Axon injury induces a transient increase in TIAR-2 granule number. The prion-like domain is necessary and sufficient for granule formation and for inhibiting regeneration. Tyrosine residues within the prion-like domain are important for granule formation and inhibition of regeneration. TIAR-2 is also serine phosphorylated in vivo. Non-phosphorylatable TIAR-2 variants do not form granules and are unable to inhibit axon regeneration. These observations suggest an in vivo function for phase-separated TIAR-2 and identify features critical for its function in axon regeneration [105]. However, there is a consensus that the regulation of a single terminal gene may not be sufficient to drive post-injury axon regeneration, especially across a long distance.
3.4.3. Alzheimer’s Disease
Alzheimer’s disease (AD) is a progressive and ultimately fatal neurocognitive disorder with behavioral disturbances characterized by brain neuron loss and deposition of misfolded proteins. Several studies have recently identified a new type of molecular pathology in AD that derives from the aggregation of RBPs, forming RNA–protein complexes that include SGs [170,171]. SGs progressively accumulate in the brains of transgenic models of tauopathy, and massively accumulate in patients with AD and other neurodegenerative diseases [170]. Some SGs (e.g., those positive for TIA1) co-localize with tau pathology, while other SGs (e.g., those positive for G3BP) often identify neurons that lack tau pathology. A significant increase in the expression of TIAR is found in the hippocampal area in AD, suggesting it could be linked with this process of neurodegeneration [83]. Further, the expression of TIAR is increased in neurons after ischemic cerebral injury [172]. However, many RBPs that are the core nucleating factors of SGs, including TIA1, TIAR, TTP and G3BP1, are also found in the pathological lesions of other neurological conditions, such as AD [94] and ischemic cerebral injury [172].
4. Future Challenges
TIAR/TIAL1 is an important multifunctional regulator of several aspects of gene expression. In this review, we included the most important discoveries related to TIAR/TIAL1 and its mechanistic implications, as well as the related cellular and pathophysiological processes. Despite the many relevant advances in recent years, there are still many questions that remain to be answered and that deserve more detailed study. For instance, the differential aspects of the a and b isoforms of TIAR have been scarcely studied. What we know to date suggests putative differential roles of both isoforms in the regulation of constitutive and alternative splicing, growth suppression, ability to act as proto-oncogenes, regulation of proliferation and cell cycles and response to damaged DNA, etc. The analysis of the interactome of RNAs and proteins associated with each of the cell-, tissue- and species-dependent TIAR isoforms would help to address these aspects. Additionally, the generation of tissue- and isoform-specific animal models with loss and gain of function would provide very useful information regarding the different activities involving TIAR isoforms. Finally, obtaining single-cell transcriptomic and proteomic expression patterns could provide novel and much more precise information concerning the role of TIAR in different cell types.
As mentioned earlier, many questions remain open about the modulation of mitochondrial activity by TIAR through, for example, NFE2L2 and other potential targets, and a comprehensive understanding of the precise mechanisms will be essential for the rational design of future clinical trials and may offer new biomarkers for monitoring therapeutic efficacy [117].
Another feature that remains poorly studied is the post-translational modification patterns of TIAR, including acetylation, methylation, phosphorylation and sumoylation, which could form another level of regulation of the protein and its isoforms and, consequently, of the processes that they modulate.
Lastly, the profiles of TIAR gene mutations should be obtained to distinguish the role of mutated variants in oncogenesis or proteostasis, as is the case of gain of function associated with tumoral and proteotoxic responses. This could also be correlated with the identification of prognostic and therapeutic targets. The ultimate and most important goal should be the development of drugs that enhance or reduce the functionality of TIAR or interacting proteins, depending on the disease, by modifying their expression patterns or biological activity. Further elucidation of the role of SGs in antiviral defense will depend on technical advances in translatome analysis and super-resolution microscopy, which have revolutionized our ability to study the composition and properties of SGs.
Acknowledgments
We are indebted to J. Alcalde and A. Fernández-Gómez for supporting and encouragement. We apologize to the many colleagues whose relevant contributions to the gene-expression and cell-biology field could not be cited because of space constraints.
Author Contributions
J.M.I. conceived the review and designed all the review. B.R.V. and J.M.I. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Research in our laboratory is supported by the Ministerio de Ciencia e Innovación and Agencia Española de Investigación through FEDER funds (RTI2018-098517-B100 and PID2021-126152OB-100) (MICINN/AEI/FEDER, UE). The CBMSO receives an institutional grant from Fundación Ramón Areces and Banco de Santander.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kawakami A., Tian Q., Duan X., Streuli M., Schlossman S.F., Anderson P. Identification and functional characterization of a TIA-1-related nucleolysin. Proc. Natl. Acad. Sci. USA. 1992;89:8681–8685. doi: 10.1073/pnas.89.18.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tian Q., Streuli M., Saito H., Schlossman S.F., Anderson P. A polyadenylate binding protein localized to the granules of cytolytic lymphocytes induces DNA fragmentation in target cells. Cell. 1991;67:629–639. doi: 10.1016/0092-8674(91)90536-8. [DOI] [PubMed] [Google Scholar]
- 3.Beck A.R., Medley Q.G., O’Brien S., Anderson P., Streuli M. Structure, tissue distribution and genomic organization of the murine RRM-type RNA binding proteins TIA-1 and TIAR. Nucleic Acids Res. 1996;24:3829–3835. doi: 10.1093/nar/24.19.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dember L.M., Kim N.D., Liu K.Q., Anderson P. Individual RNA recognition motifs of TIA-1 and TIAR have different RNA binding specificities. J. Biol. Chem. 1996;271:2783–2788. doi: 10.1074/jbc.271.5.2783. [DOI] [PubMed] [Google Scholar]
- 5.Kim H.S., Headey S.J., Yoga Y.M., Scanlon M.J., Gorospe M., Wilce M.C., Wilce J.A. Distinct binding properties of TIAR RRMs and linker region. RNA Biol. 2013;10:579–589. doi: 10.4161/rna.24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waris S., Wilce M.C., Wilce J.A. RNA recognition and stress granule formation by TIA proteins. Int. J. Mol. Sci. 2014;15:23377–23388. doi: 10.3390/ijms151223377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taupin J.L., Tian Q., Kedersha N., Robertson M., Anderson P. The RNA-binding protein TIAR is translocated from the nucleus to the cytoplasm during Fas-mediated apoptotic cell death. Proc. Natl. Acad. Sci. USA. 1995;92:1629–1633. doi: 10.1073/pnas.92.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernández-Gómez A., Izquierdo J.M. The multifunctional faces of T-cell Intracellular antigen 1 in health and disease. Int. J. Mol. Sci. 2022;23:1400. doi: 10.3390/ijms23031400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adam S.A., Nakagawa T., Swanson M.S., Woodruff T.K., Dreyfuss G. mRNA polyadenylate-binding protein: Gene isolation and sequencing and identification of a ribonucleoprotein consensus sequence. Mol. Cell. Biol. 1986;6:2932–2943. doi: 10.1128/mcb.6.8.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swanson M.S., Nakagawa T.Y., LeVan K., Dreyfuss G. Primary structure of human nuclear ribonucleoprotein particle C proteins: Conservation of sequence and domain structures in heterogeneous nuclear RNA, mRNA, and pre-rRNA-binding proteins. Mol. Cell. Biol. 1987;7:173–1739. doi: 10.1128/mcb.7.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dreyfuss G., Swanson M.S., Pinol-Roma S. Heterogeneous nuclear ribonucleoprotein particles and the pathway of mRNA formation. Trends Biochem. Sci. 1988;13:86–91. doi: 10.1016/0968-0004(88)90046-1. [DOI] [PubMed] [Google Scholar]
- 12.Mazan-Mamczarz K., Lal A., Martindale J.L., Kawai T., Gorospe M. Translational repression by RNA-binding protein TIAR. Mol. Cell. Biol. 2006;26:2716–2727. doi: 10.1128/MCB.26.7.2716-2727.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim H.S., Kuwano Y., Zhan M., Pullmann R., Jr., Mazan-Mamczarz K., Li H., Kedersha N., Anderson P., Wilce M.C., Gorospe M., et al. Elucidation of a C-rich signature motif in target mRNAs of RNA-binding protein TIAR. Mol. Cell. Biol. 2007;27:6806–6817. doi: 10.1128/MCB.01036-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izquierdo J.M., Valcárcel J. Two isoforms of the T-cell intracellular antigen 1 (TIA-1) splicing factor display distinct splicing regulation activities. Control of TIA-1 isoform ratio by TIA-1-related protein. J. Biol. Chem. 2007;282:19410–19417. doi: 10.1074/jbc.M700688200. [DOI] [PubMed] [Google Scholar]
- 15.The Human Protein Atlas. [(accessed on 20 May 2022)]. Available online: https://www.proteinatlas.org/ENSG00000151923-TIAL1.
- 16.Eraslan G., Drokhlyansky E., Anand S., Fiskin E., Subramanian A., Slyper M., Wang J., Van Wittenberghe N., Rouhana J.M., Julia Waldman J., et al. Single-nucleus cross-tissue molecular reference maps toward understanding disease gene function. Science. 2022;376:eabl4290. doi: 10.1126/science.abl4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabula Sapiens Consortium. Jones R.C., Karkanias J., Krasnow M.A., Oliveira Pisco A., Quake S.R., Salzman J., Yosef N., Bulthaup B., Brown P., et al. The Tabula Sapiens: A multiple-organ, single-cell transcriptomic atlas of humans. Science. 2022;376:eabl4896. doi: 10.1126/science.abl4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Domínguez Conde C., Xu C., Jarvis L.B., Rainbow D.B., Wells S.B., Gomes T., Howlett S.K., Suchanek O., Polanski K., King H.W., et al. Cross-tissue immune cell analysis reveals tissue-specific features in humans. Science. 2022;376:eabl5197. doi: 10.1126/science.abl5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pullmann R., Jr., Kim H.H., Abdelmohsen K., Lal A., Martindale J.L., Yang X., Gorospe M. Analysis of turnover and translation regulatory RNA-binding protein expression through binding to cognate mRNAs. Mol. Cell. Biol. 2007;27:6265–6278. doi: 10.1128/MCB.00500-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masuda K., Marasa B., Martindale J.L., Halushka M.K., Gorospe M. Tissue- and age-dependent expression of RNA-binding proteins that influence mRNA turnover and translation. Aging. 2009;1:681–698. doi: 10.18632/aging.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho N.H., Cheveralls K.C., Brunner A.D., Kim K., Michaelis A.C., Raghavan P., Kobayashi H., Savy L., Li J.Y., Canaj H., et al. OpenCell: Endogenous tagging for the cartography of human cellular organization. Science. 2022;375:eabi6983. doi: 10.1126/science.abi6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das R., Yu J., Zhang Z., Gygi M.P., Krainer A.R., Gygi S.P., Reed R. SR proteins function in coupling RNAP II transcription to pre-mRNA splicing. Mol. Cell. 2007;26:867–881. doi: 10.1016/j.molcel.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 23.Suswam E.A., Li Y.Y., Mahtani H., King P.H. Novel DNA-binding properties of the RNA-binding protein TIAR. Nucleic Acids Res. 2005;33:4507–4518. doi: 10.1093/nar/gki763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim H.S., Wilce M.C., Yoga Y.M., Pendini N.R., Gunzburg M.J., Cowieson N.P., Wilson G.M., Williams B.R., Gorospe M., Wilce J.A. Different modes of interaction by TIAR and HuR with target RNA and DNA. Nucleic Acids Res. 2011;39:1117–1130. doi: 10.1093/nar/gkq837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tominaga A., Sugawara H., Futagawa T., Inoue K., Sasaki K., Minamino N., Hatakeyama M., Handa H., Miyata A. Characterization of the testis-specific promoter region in the human pituitary adenylate cyclase-activating polypeptide (PACAP) gene. Genes Cells. 2010;15:595–606. doi: 10.1111/j.1365-2443.2010.01403.x. [DOI] [PubMed] [Google Scholar]
- 26.Reyes R., Alcalde J., Izquierdo J.M. Depletion of T-cell intracellular antigen proteins promotes cell proliferation. Genome Biol. 2009;10:R87. doi: 10.1186/gb-2009-10-8-r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Del Gatto-Konczak F., Bourgeois C.F., Le Guiner C., Kister L., Gesnel M.C., Stévenin J., Breathnach R. The RNA-binding protein TIA-1 is a novel mammalian splicing regulator acting through intron sequences adjacent to a 5′ splice site. Mol. Cell. Biol. 2000;20:6287–6299. doi: 10.1128/MCB.20.17.6287-6299.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Förch P., Puig O., Kedersha N., Martínez C., Granneman S., Séraphin B., Anderson P., Valcárcel J. The apoptosis-promoting factor TIA-1 is a regulator of alternative pre-mRNA splicing. Mol. Cell. 2000;6:1089–1098. doi: 10.1016/S1097-2765(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 29.Le Guiner C., Lejeune F., Galiana D., Kister L., Breathnach R., Stévenin J., Del Gatto-Konczak F. TIA-1 and TIAR activate splicing of alternative exons with weak 5’ splice sites followed by a U-rich stretch on their own pre-mRNAs. J. Biol. Chem. 2001;276:40638–40646. doi: 10.1074/jbc.M105642200. [DOI] [PubMed] [Google Scholar]
- 30.Förch P., Puig O., Martínez C., Séraphin B., Valcárcel J. The splicing regulator TIA-1 interacts with U1-C to promote U1 snRNP recruitment to 5′ splice sites. EMBO J. 2002;21:6882–6892. doi: 10.1093/emboj/cdf668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Izquierdo J.M., Majós N., Bonnal S., Martínez C., Castelo R., Guigó R., Bilbao D., Valcárcel J. Regulation of Fas alternative splicing by antagonistic effects of TIA-1 and PTB on exon definition. Mol. Cell. 2005;19:475–484. doi: 10.1016/j.molcel.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Zhu H., Hinman M.N., Hasman R.A., Mehta P., Lou H. Regulation of neuron-specific alternative splicing of neurofibromatosis type 1 pre-mRNA. Mol. Cell. Biol. 2008;28:1240–1251. doi: 10.1128/MCB.01509-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aznarez I., Barash Y., Shai O., He D., Zielenski J., Tsui L.C., Parkinson J., Frey B.J., Rommens J.M., Blencowe B.J. A systematic analysis of intronic sequences downstream of 5′ splice sites reveals a widespread role for U-rich motifs and TIA1/TIAL1 proteins in alternative splicing regulation. Genome Res. 2008;18:1247–1258. doi: 10.1101/gr.073155.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gal-Mark N., Schwartz S., Ram O., Eyras E., Ast G. The pivotal roles of TIA proteins in 5′ splice-site selection of alu exons and across evolution. PLoS Genet. 2009;5:e1000717. doi: 10.1371/journal.pgen.1000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z., Kayikci M., Briese M., Zarnack K., Luscombe N.M., Rot G., Zupan B., Curk T., Ule J. iCLIP predicts the dual splicing effects of TIA-RNA interactions. PLoS Biol. 2010;8:1000530. doi: 10.1371/journal.pbio.1000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Izquierdo J.M. Heterogeneous ribonucleoprotein C displays a repressor activity mediated by T-cell intracellular antigen-1-related/like protein to modulate Fas exon 6 splicing through a mechanism involving Hu antigen R. Nucleic Acids Res. 2010;38:8001–8014. doi: 10.1093/nar/gkq698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh N.N., Seo J., Ottesen E.W., Shishimorova M., Bhattacharya D., Singh R.N. TIA1 prevents skipping of a critical exon associated with spinal muscular atrophy. Mol. Cell. Biol. 2011;31:935–954. doi: 10.1128/MCB.00945-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer C., Garzia A., Mazzola M., Gerstberger S., Molina H., Tuschl T. The TIA1 RNA-binding protein family regulates EIF2AK2-mediated stress response and cell cycle progression. Mol. Cell. 2018;69:622–635. doi: 10.1016/j.molcel.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Nostrand E.L., Freese P., Pratt G.A., Wang X., Wei X., Xiao R., Blue S.M., Chen J.Y., Cody N.A.L., Dominguez D., et al. A large-scale binding and functional map of human RNA-binding proteins. Nature. 2020;583:711–719. doi: 10.1038/s41586-020-2077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao W., Zhao J., Hou M., Wang Y., Zhang Y., Zhao X., Zhang C., Guo D. HuR and TIA1/TIAL1 are involved in regulation of alternative splicing of SIRT1 pre-mRNA. Int. J. Mol. Sci. 2014;15:2946–2958. doi: 10.3390/ijms15022946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Královičová J., Borovská I., Pengelly R., Lee E., Abaffy P., Šindelka R., Grutzner F., Vořechovský I. Restriction of an intron size en route to endothermy. Nucleic Acids Res. 2021;49:2460–2487. doi: 10.1093/nar/gkab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu H., Hasman R.A., Young K.M., Kedersha N.L., Hua L. U1 snRNP-dependent function of TIAR in the regulation of alternative RNA processing of the human calcitonin/CGRP pre-mRNA. Mol. Cell. Biol. 2003;23:5959–5971. doi: 10.1128/MCB.23.17.5959-5971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shukla S., Dirksen W.P., Joyce K.M., Le Guiner-Blanvillain C., Breathnach R., Fisher S.A. TIA proteins are necessary but not sufficient for the tissue-specific splicing of the myosin phosphatase targeting subunit 1. J. Biol. Chem. 2004;279:13668–13676. doi: 10.1074/jbc.M314138200. [DOI] [PubMed] [Google Scholar]
- 44.Zhang T., Delestienne N., Huez G., Kruys V., Gueydan C. Identification of the sequence determinants mediating the nucleo-cytoplasmic shuttling of TIAR and TIA-1 RNA-binding proteins. J. Cell. Sci. 2005;118:5453–5463. doi: 10.1242/jcs.02669. [DOI] [PubMed] [Google Scholar]
- 45.Gueydan C., Droogmans L., Chalon P., Huez G., Caput D., Kruys V. Identification of TIAR as a protein binding to the translational regulatory AU-rich element of tumor necrosis factor alpha mRNA. J. Biol. Chem. 1999;274:2322–2326. doi: 10.1074/jbc.274.4.2322. [DOI] [PubMed] [Google Scholar]
- 46.Piecyk M., Wax S., Beck A.R., Kedersha N., Gupta M., Maritim B., Chen S., Gueydan C., Kruys V., Streuli M., et al. TIA-1 is a translational silencer that selectively regulates the expression of TNF-alpha. EMBO J. 2000;19:4154–4163. doi: 10.1093/emboj/19.15.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu Q., Cok S.J., Zeng C., Morrison A.R. Translational repression of human matrix metalloproteinases-13 by an alternatively spliced form of T-cell-restricted intracellular antigen-related protein (TIAR) J. Biol. Chem. 2003;278:1579–1584. doi: 10.1074/jbc.M203526200. [DOI] [PubMed] [Google Scholar]
- 48.Cok S.J., Acton S.J., Morrison A.R. The proximal region of the 3’-untranslated region of cyclooxygenase-2 is recognized by a multimeric protein complex containing HuR, TIA-1, TIAR, and the heterogeneous nuclear ribonucleoprotein U. J. Biol. Chem. 2003;278:36157–36162. doi: 10.1074/jbc.M302547200. [DOI] [PubMed] [Google Scholar]
- 49.Cok S.J., Acton S.J., Sexton A.E., Morrison A.R. Identification of RNA-binding proteins in RAW 264.7 cells that recognize a lipopolysaccharide-responsive element in the 3-untranslated region of the murine cyclooxygenase-2 mRNA. J. Biol. Chem. 2004;279:8196–8205. doi: 10.1074/jbc.M308475200. [DOI] [PubMed] [Google Scholar]
- 50.Kandasamy K., Joseph K., Subramaniam K., Raymond J.R., Tholanikunnel B.G. Translational control of beta2-adrenergic receptor mRNA by T-cell-restricted intracellular antigen-related protein. J. Biol. Chem. 2005;280:1931–1943. doi: 10.1074/jbc.M405937200. [DOI] [PubMed] [Google Scholar]
- 51.László C.F., Fayad S., Carpenter O., George K.S., Lu W., Saad A.A., Wu S. The role of translational regulation in ultraviolet C light-induced cyclooxygenase-2 expression. Life Sci. 2009;85:70–76. doi: 10.1016/j.lfs.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Subramaniam K., Kandasamy K., Joseph K., Spicer E.K., Tholanikunnel B.G. The 3′-untranslated region length and AU-rich RNA location modulate RNA-protein interaction and translational control of β2-adrenergic receptor mRNA. Mol. Cell. Biochem. 2011;352:125–141. doi: 10.1007/s11010-011-0747-z. [DOI] [PubMed] [Google Scholar]
- 53.Colegrove-Otero L.J., Devaux A., Standart N. The Xenopus ELAV protein ElrB represses Vg1 mRNA translation during oogenesis. Mol. Cell. Biol. 2005;25:9028–9039. doi: 10.1128/MCB.25.20.9028-9039.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lal A., Abdelmohsen K., Pullmann R., Kawai T., Galban S., Yang X., Brewer G., Gorospe M. Posttranscriptional derepression of GADD45alpha by genotoxic stress. Mol. Cell. 2006;22:117–128. doi: 10.1016/j.molcel.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 55.Yu C., York B., Wang S., Feng Q., Xu J., O’Malley B.W. An essential function of the SRC-3 coactivator in suppression of cytokine mRNA translation and inflammatory response. Mol. Cell. 2007;25:765–778. doi: 10.1016/j.molcel.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liao B., Hu Y., Brewer G. Competitive binding of AUF1 and TIAR to MYC mRNA controls its translation. Nat. Struct. Mol. Biol. 2007;14:511–518. doi: 10.1038/nsmb1249. [DOI] [PubMed] [Google Scholar]
- 57.Damgaard C.K., Lykke-Andersen J. Translational coregulation of 5’TOP mRNAs by TIA-1 and TIAR. Genes Dev. 2011;25:2057–2068. doi: 10.1101/gad.17355911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miloslavski R., Cohen E., Avraham A., Iluz Y., Hayouka Z., Kasir J., Mudhasani R., Jones S.N., Cybulski N., Rüegg M.A., et al. Oxygen sufficiency controls TOP mRNA translation via the TSC-Rheb-mTOR pathway in a 4E-BP-independent manner. J. Mol. Cell. Biol. 2014;6:255–266. doi: 10.1093/jmcb/mju008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fred R.G., Mehrabi S., Adams C.M., Welsh N. PTB and TIAR binding to insulin mRNA 3’- and 5’-UTRs; implications for insulin biosynthesis and messenger stability. Heliyon. 2016;2:e00159. doi: 10.1016/j.heliyon.2016.e00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marchese D., Botta-Orfila T., Cirillo D., Rodriguez J.A., Livi C.M., Fernández-Santiago R., Ezquerra M., Martí M.J., Bechara E., Tartaglia G.G., et al. Registry (CMSAR). Discovering the 3′ UTR-mediated regulation of alpha-synuclein. Nucleic Acids Res. 2017;45:12888–12903. doi: 10.1093/nar/gkx1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carrascoso I., Sánchez-Jiménez C., Izquierdo J.M. Genome-wide profiling reveals a role for T-cell intracellular antigens TIA1 and TIAR in the control of translational specificity in HeLa cells. Biochem. J. 2014;461:43–50. doi: 10.1042/BJ20140227. [DOI] [PubMed] [Google Scholar]
- 62.Carrascoso I., Sánchez-Jiménez C., Izquierdo J.M. Long-term reduction of T-cell intracellular antigens leads to increased beta-actin expression. Mol. Cancer. 2014;13:90. doi: 10.1186/1476-4598-13-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baez M.V., Boccaccio G.L. Mammalian Smaug is a translational repressor that forms cytoplasmic foci similar to stress granules. J. Biol. Chem. 2005;280:43131–43140. doi: 10.1074/jbc.M508374200. [DOI] [PubMed] [Google Scholar]
- 64.Connolly E.P., Thuillier V., Rouy D., Bouétard G., Schneider R.J. Inhibition of Cap-initiation complexes linked to a novel mechanism of eIF4G depletion in acute myocardial ischemia. Cell Death. Differ. 2006;13:1586–1594. doi: 10.1038/sj.cdd.4401854. [DOI] [PubMed] [Google Scholar]
- 65.Reinhardt H.C., Hasskamp P., Schmedding I., Morandell S., van Vugt M.A., Wang X., Linding R., Ong S.E., Weaver D., Carr S.A., et al. DNA damage activates a spatially distinct late cytoplasmic cell-cycle checkpoint network controlled by MK2-mediated RNA stabilization. Mol. Cell. 2010;40:34–49. doi: 10.1016/j.molcel.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Velázquez-Cruz A., Baños-Jaime B., Díaz-Quintana A., De la Rosa M.A., Díaz-Moreno I. Post-translational control of RNA-binding proteins and disease-related dysregulation. Front. Mol. Biosci. 2021;8:658852. doi: 10.3389/fmolb.2021.658852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dean J.L., Sully G., Clark A.R., Saklatvala J. The involvement of AU-rich element-binding proteins in p38 mitogen-activated protein kinase pathway-mediated mRNA stabilisation. Cell Signal. 2004;16:1113–1121. doi: 10.1016/j.cellsig.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 68.Duttagupta R., Tian B., Wilusz C.J., Khounh D.T., Soteropoulos P., Ouyang M., Dougherty J.P., Peltz S.W. Global analysis of Pub1p targets reveals a coordinate control of gene expression through modulation of binding and stability. Mol. Cell. Biol. 2005;25:5499–5513. doi: 10.1128/MCB.25.13.5499-5513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.García-Mauriño S.M., Rivero-Rodríguez F., Velázquez-Cruz A., Hernández-Vellisca M., Díaz-Quintana A., De la Rosa M.A., Díaz-Moreno I. RNA binding protein regulation and cross-talk in the control of AU-rich mRNA fate. Front. Mol. Biosci. 2017;4:71. doi: 10.3389/fmolb.2017.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fechir M., Linker K., Pautz A., Hubrich T., Kleinert H. The RNA binding protein TIAR is involved in the regulation of human iNOS expression. Cell. Mol. Biol. 2005;51:299–305. [PubMed] [Google Scholar]
- 71.Motohashi H., Mukudai Y., Ito C., Kato K., Shimane T., Kondo S., Shirota T. Tumor protein D52 expression is post-transcriptionally regulated by T-cell intercellular antigen (TIA) 1 and TIA-related protein via mRNA stability. Biochem. J. 2017;474:1669–1687. doi: 10.1042/BCJ20160942. [DOI] [PubMed] [Google Scholar]
- 72.Stoecklin G., Stubbs T., Kedersha N., Wax S., Rigby W.F., Blackwell T.K., Anderson P. MK2-induced tristetraprolin: 14-3-3 complexes prevent stress granule association and ARE-mRNA decay. EMBO J. 2004;23:1313–1324. doi: 10.1038/sj.emboj.7600163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo X., Sun Y., Azad T., Janse van Rensburg H.J., Luo J., Yang S., Liu P., Lv Z., Zhan M., Lu L., et al. Rox8 promotes microRNA-dependent yki messenger RNA decay. Proc. Natl. Acad. Sci. USA. 2020;117:30520–30530. doi: 10.1073/pnas.2013449117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu L., Yue H., Liu Q., Yuan J., Li J., Wei G., Chen X., Lu Y., Guo M., Luo J., et al. LncRNA MT1JP functions as a tumor suppressor by interacting with TIAR to modulate the p53 pathway. Oncotarget. 2016;7:15787–15800. doi: 10.18632/oncotarget.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu A.T., Berasain C., Bhatia S., Rivera K., Liu B., Rigo F., Pappin D.J., Spector D.L. PHAROH lncRNA regulates Myc translation in hepatocellular carcinoma via sequestering TIAR. eLife. 2021;10:e68263. doi: 10.7554/eLife.68263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yi B., Li H., Cai H., Lou X., Yu M., Li Z. LOXL1-AS1 communicating with TIAR modulates vasculogenic mimicry in glioma via regulation of the miR-374b-5p/MMP14 axis. J. Cell. Mol. Med. 2022;26:475–490. doi: 10.1111/jcmm.17106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suvanto M., Beesley J., Blomqvist C., Chenevix-Trench G., Khan S., Nevanlinna H. SNPs in lncRNA regions and breast cancer risk. Front. Genet. 2020;11:550. doi: 10.3389/fgene.2020.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Q., Yu H., Yu H., Ma M., Ma Y., Li R. miR-223-3p/TIAL1 interaction is involved in the mechanisms associated with the neuroprotective effects of dexmedetomidine on hippocampal neuronal cells in vitro. Mol. Med. Rep. 2019;19:805–812. doi: 10.3892/mmr.2018.9742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.El Gazzar M., McCall C.E. MicroRNAs distinguish translational from transcriptional silencing during endotoxin tolerance. J. Biol. Chem. 2010;285:20940–20951. doi: 10.1074/jbc.M110.115063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Collino F., Deregibus M.C., Bruno S., Sterpone L., Aghemo G., Viltono L., Tetta C., Camussi G. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS ONE. 2010;5:e11803. doi: 10.1371/journal.pone.0011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beck A.R., Miller I.J., Anderson P., Streuli M. RNA-binding protein TIAR is essential for primordial germ cell development. Proc. Natl. Acad. Sci. USA. 1998;95:2331–2336. doi: 10.1073/pnas.95.5.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oleana V.H., Salehi A., Swaab D.F. Increased expression of the TIAR protein in the hippocampus of Alzheimer patients. NeuroReport. 1998;9:1451–1454. doi: 10.1097/00001756-199805110-00037. [DOI] [PubMed] [Google Scholar]
- 83.Kedersha N.L., Gupta M., Li W., Miller I., Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J. Cell. Biol. 1999;147:1431–1442. doi: 10.1083/jcb.147.7.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kedersha N., Chen S., Gilks N., Li W., Miller I.J., Stahl J., Anderson P. Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol. Biol. Cell. 2002;13:195–210. doi: 10.1091/mbc.01-05-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iseni F., Garcin D., Nishio M., Kedersha N., Anderson P., Kolakofsky D. Sendai virus trailer RNA binds TIAR, a cellular protein involved in virus-induced apoptosis. EMBO J. 2002;21:5141–5150. doi: 10.1093/emboj/cdf513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li W., Li Y., Kedersha N., Anderson P., Emara M., Swiderek K.M., Moreno G.T., Brinton M.A. Cell proteins TIA-1 and TIAR interact with the 3’ stem-loop of the West Nile virus complementary minus-strand RNA and facilitate virus replication. J. Virol. 2002;76:11989–12000. doi: 10.1128/JVI.76.23.11989-12000.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Le Guiner C., Gesnel M.C., Breathnach R. TIA-1 or TIAR is required for DT40 cell viability. J. Biol. Chem. 2003;278:10465–10476. doi: 10.1074/jbc.M212378200. [DOI] [PubMed] [Google Scholar]
- 88.Emara M.M., Brinton M.A. Interaction of TIA-1/TIAR with West Nile and dengue virus products in infected cells interferes with stress granule formation and processing body assembly. Proc. Natl. Acad. Sci. USA. 2007;104:9041–9046. doi: 10.1073/pnas.0703348104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Emara M.M., Liu H., Davis W.G., Brinton M.A. Mutation of mapped TIA-1/TIAR binding sites in the 3’ terminal stem-loop of West Nile virus minus-strand RNA in an infectious clone negatively affects genomic RNA amplification. J. Virol. 2008;82:10657–10670. doi: 10.1128/JVI.00991-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kharraz Y., Salmand P.-A., Camus A., Auriol J., Gueydan C., Kruys V., Morello D. Impaired embryonic development in mice overexpressing the RNA-binding protein TIAR. PLoS ONE. 2010;5:e11352. doi: 10.1371/journal.pone.0011352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Izquierdo J.M., Alcalde J., Carrascoso I., Reyes R., Ludeña M.D. Knockdown of T-cell intracellular antigens triggers cell proliferation, invasion and tumour growth. Biochem. J. 2011;435:337–344. doi: 10.1042/BJ20101030. [DOI] [PubMed] [Google Scholar]
- 92.Garaigorta U., Heim M.H., Boyd B., Wieland S., Chisari F.V. Hepatitis C virus (HCV) induces formation of stress granules whose proteins regulate HCV RNA replication and virus assembly and egress. J. Virol. 2012;86:11043–11056. doi: 10.1128/JVI.07101-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sánchez-Jiménez C., Izquierdo J.M. T-cell intracellular antigen (TIA)-proteins deficiency in murine embryonic fibroblasts alters cell cycle progression and induces autophagy. PLoS ONE. 2013;8:e75127. doi: 10.1371/journal.pone.0075127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ash P.E., Vanderweyde T.E., Youmans K.L., Apicco D.J., Wolozin B. Pathological stress granules in Alzheimer’s disease. Brain Res. 2014;1584:52–58. doi: 10.1016/j.brainres.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sánchez-Jiménez C., Ludeña M.D., Izquierdo J.M. T-cell intracellular antigens function as tumor suppressor genes. Cell Death Dis. 2015;6:e1669. doi: 10.1038/cddis.2015.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bley N., Lederer M., Pfalz B., Reinke C., Fuchs T., Glaß M., Möller B., Hüttelmaier S. Stress granules are dispensable for mRNA stabilization during cellular stress. Nucleic Acids Res. 2015;43:e26. doi: 10.1093/nar/gku1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Geng Z., Li P., Tan L., Song H. Targeted knockdown of RNA-binding protein TIAR for promoting self-renewal and attenuating differentiation of mouse embryonic stem cells. Stem Cells Int. 2015;2015:657325. doi: 10.1155/2015/657325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kharraz Y., Lefort A., Libert F., Christopher J., Mann C.J., Gueydan C., Kruys V. Genome-wide analysis of TIAR RNA ligands in mouse macrophages before and after LPS stimulation. Genom. Data. 2016;7:297–300. doi: 10.1016/j.gdata.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tak H., Eun J.W., Kim J., Park S.J., Kim C., Ji E., Lee H., Kang H., Cho D.H., Lee K., et al. T-cell-restricted intracellular antigen 1 facilitates mitochondrial fragmentation by enhancing the expression of mitochondrial fission factor. Cell Death Differ. 2017;24:49–58. doi: 10.1038/cdd.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carrascoso I., Alcalde J., Sánchez-Jiménez C., González-Sánchez P., Izquierdo J.M. T-cell intracellular antigens and Hu antigen R antagonistically modulate mitochondrial activity and dynamics by regulating optic atrophy 1 gene expression. Mol. Cell. Biol. 2017;37:e0017417. doi: 10.1128/MCB.00174-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hou S., Kumar A., Xu Z., Airo A.M., Stryapunina I., Wong C.P., Branton W., Tchesnokov E., Götte M., Power C., et al. Zika virus hijacks stress granule proteins and modulates the host stress response. J. Virol. 2017;91:e00474-17. doi: 10.1128/JVI.00474-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Battu S., Afroz S., Giddaluru J., Naz S., Huang W., Khumukcham S.S., Khan R.A., Bhat S.Y., Qureshi I.A., Manavathi B., et al. Amino acid starvation sensing dampens IL-1β production by activating riboclustering and autophagy. PLoS Biol. 2018;16:e2005317. doi: 10.1371/journal.pbio.2005317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Naz S., Khan R.A., Giddaluru J., Battu S., Vishwakarma S.K., Subahan M., Satti V., Khan N., Khan A.A. Transcriptome meta-analysis identifies immune signature comprising of RNA binding proteins in ulcerative colitis patients. Cell Immunol. 2018;334:42–48. doi: 10.1016/j.cellimm.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 104.Lafarga V., Sung H.-M., Haneke K., Roessig L., Pauleau A.-L., Bruer M., Rodriguez-Acebes S., Lopez-Contreras A.J., Gruss O.J., Erhardt S., et al. TIAR marks nuclear G2/M transition granules and restricts CDK1 activity under replication stress. EMBO Rep. 2019;20:e46224. doi: 10.15252/embr.201846224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Andrusiak M.G., Sharifnia P., Lyu X., Wang Z., Dickey A.M., Wu Z., Chisholm A.D., Jin Y. Inhibition of axon regeneration by liquid-like TIAR-2 granules. Neuron. 2019;104:290–304. doi: 10.1016/j.neuron.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mahboubi H., Moujaber O., Kodiha M., Stochaj U. The co-chaperone HspBP1 is a novel component of stress granules that regulates their formation. Cells. 2020;9:825. doi: 10.3390/cells9040825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carrascoso I., Alcalde J., Tabas-Madrid D., Oliveros J.C., Izquierdo J.M. Transcriptome-wide analysis links the short-term expression of the b isoforms of TIA proteins to protective proteostasis-mediated cell quiescence response. PLoS ONE. 2018;13:e0208526. doi: 10.1371/journal.pone.0208526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Naz S., Battu S., Khan R.A., Afroz S., Giddaluru J., Vishwakarma S.K., Satti V., Habeeb M.A., Khan A.A., Khan N. Activation of integrated stress response pathway regulates IL-1β production through posttranscriptional and translational reprogramming in macrophages. Eur. J. Immunol. 2019;49:277–289. doi: 10.1002/eji.201847513. [DOI] [PubMed] [Google Scholar]
- 109.Ostareck D.H., Ostareck-Lederer A. RNA-binding proteins in the control of LPS-induced macrophage response. Front. Genet. 2019;10:31. doi: 10.3389/fgene.2019.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Silva-García C.G., Estela Navarro R. The C. elegans TIA-1/TIAR homolog TIAR-1 is required to induce germ cell apoptosis. Genesis. 2013;51:690–707. doi: 10.1002/dvg.22418. [DOI] [PubMed] [Google Scholar]
- 111.Bossowski A., Czarnocka B., Bardadin K., Moniuszko A., Łyczkowska A., Czerwinska J., Dadan J., Bossowska A. Identification of chosen apoptotic (TIAR and TIA1) markers expression in thyroid tissues from adolescents with immune and non-immune thyroid diseases. Folia Histochem. Cytobiol. 2010;48:178–184. doi: 10.2478/v10042-010-0022-2. [DOI] [PubMed] [Google Scholar]
- 112.Lu L., Wang S., Zheng L., Li X., Suswam E.A., Zhang X., Wheeler C.G., Nabors L.B., Filippova N., King P.H. Amyotrophic lateral sclerosis-linked mutant SOD1 sequesters Hu antigen R (HuR) and TIA-1-related protein (TIAR): Implications for impaired post-transcriptional regulation of vascular endothelial growth factor. J. Biol. Chem. 2009;284:33989–33998. doi: 10.1074/jbc.M109.067918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu H., Sun H., Zhang H., Liu J., Fan F., Li Y., Ning X., Sun Y., Dai S., Liu B., et al. An shRNA based genetic screen identified Sesn2 as a potential tumor suppressor in lung cancer via suppression of Akt-mTOR-p70S6K signaling. PLoS ONE. 2015;10:e0124033. doi: 10.1371/journal.pone.0124033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ravanidis S., Doxakis E. RNA-binding proteins implicated in mitochondrial damage and mitophagy. Front. Cell. Dev. Biol. 2020;8:372. doi: 10.3389/fcell.2020.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Carrascoso I., Sánchez-Jiménez C., Silion E., Alcalde J., Izquierdo J.M. A heterologous cell model for studying the role of T-cell intracellular antigen 1 in Welander distal myopathy. Mol. Cell. Biol. 2019;39:e0029918. doi: 10.1128/MCB.00299-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yongjie Cen Y., Zou X., Zhong Q., Chen Y., Lin Y., Feng Q., Wang X., Zheng S. The TIAR-mediated Nrf2 response to oxidative stress is mediated through the Nrf2 noncoding 3’untranslated region in Spodoptera litura. Free Radic. Biol. Med. 2022;184:17–29. doi: 10.1016/j.freeradbiomed.2022.03.016. [DOI] [PubMed] [Google Scholar]
- 117.Dinkova-Kostovaa A.T., Abramovc A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med. 2015;88:179–188. doi: 10.1016/j.freeradbiomed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.MSGP—Database. [(accessed on 20 May 2022)]. Available online: https://msgp.pt/
- 119.RNP Granule Database. [(accessed on 20 May 2022)]. Available online: https://rnagranuledb.lunenfeld.ca/
- 120.Esclatine A., Taddeo B., Roizman B. Herpes simplex virus 1 induces cytoplasmic accumulation of TIA-1/TIAR and both synthesis and cytoplasmic accumulation of tristetraprolin, two cellular proteins that bind and destabilize AU-rich RNAs. J. Virol. 2004;78:8582–8592. doi: 10.1128/JVI.78.16.8582-8592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thomas M.G., Martinez Tosar L.J., Loschi M., Pasquini J.M., Correale J., Kindler S., Boccaccio G.L. Staufen recruitment into stress granules does not affect early mRNA transport in oligodendrocytes. Mol. Biol. Cell. 2005;16:405–420. doi: 10.1091/mbc.e04-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sampuda K.M., Riley M., Boyd L. Stress induced nuclear granules form in response to accumulation of misfolded proteins in Caenorhabditis elegans. BMC Cell Biol. 2017;18:18. doi: 10.1186/s12860-017-0136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wiegand M., Bossow S., Neubert W.J. Sendai virus trailer RNA simultaneously blocks two apoptosis-inducing mechanisms in a cell type-dependent manner. J. Gen. Virol. 2005;86:2305–2314. doi: 10.1099/vir.0.81022-0. [DOI] [PubMed] [Google Scholar]
- 124.Moujaber O., Mahboubi H., Kodiha M., Bouttier M., Bednarz K., Bakshi R., White J., Larose L., Colmegna I., Stochaj U. Dissecting the molecular mechanisms that impair stress granule formation in aging cells. Biochim. Biophys. Acta Mol. Cell. Res. 2017;1864:475–486. doi: 10.1016/j.bbamcr.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 125.Delestienne N., Wauquier C., Soin R., Dierick J.F., Gueydan C., Kruys V. The splicing factor ASF/SF2 is associated with TIA-1-related/TIA-1-containing ribonucleoproteic complexes and contributes to post-transcriptional repression of gene expression. FEBS J. 2010;277:2496–2514. doi: 10.1111/j.1742-4658.2010.07664.x. [DOI] [PubMed] [Google Scholar]
- 126.Tessier S.N., Audas T.E., Wu C.W., Lee S., Storey K.B. The involvement of mRNA processing factors TIA-1, TIAR, and PABP-1 during mammalian hibernation. Cell Stress Chaperones. 2014;19:813–825. doi: 10.1007/s12192-014-0505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Weeks A., Agnihotri S., Lymer J., Chalil A., Diaz R., Isik S., Smith C., Rutka J.T. Epithelial cell transforming 2 and aurora kinase B modulate formation of stress granule-containing transcripts from diverse cellular pathways in astrocytoma cells. Am. J. Pathol. 2016;186:1674–1687. doi: 10.1016/j.ajpath.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 128.Palangi F., Samuel S.M., Thompson I.R., Triggle C.R., Emara M.M. Effects of oxidative and thermal stresses on stress granule formation in human induced pluripotent stem cells. PLoS ONE. 2017;12:e0182059. doi: 10.1371/journal.pone.0182059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lechler M.C., David D.C. More stressed out with age? Check your RNA granule aggregation. Prion. 2017;11:313–322. doi: 10.1080/19336896.2017.1356559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kuo C.T., You G.T., Jian Y.J., Chen T.S., Siao Y.C., Hsu A.L., Ching T.T. AMPK-mediated formation of stress granules is required for dietary restriction-induced longevity in Caenorhabditis elegans. Aging Cell. 2020;19:e13157. doi: 10.1111/acel.13157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lo Bello M., Di Fini F., Notaro A., Spataro R., Conforti F.L., La Bella V. ALS-related mutant FUS protein is mislocalized to cytoplasm and is recruited into stress granules of fibroblasts from asymptomatic FUS P525L mutation carriers. Neurodegener. Dis. 2017;17:292–303. doi: 10.1159/000480085. [DOI] [PubMed] [Google Scholar]
- 132.Buddika K., Ariyapala I.S., Hazuga M.A., Riffert D., Sokol N.S. Canonical nucleators are dispensable for stress granule assembly in Drosophila intestinal progenitors. J. Cell. Sci. 2020;133:jcs243451. doi: 10.1242/jcs.243451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Aulas A., Finetti P., Lyons S.M., Bertucci F., Birnbaum D., Acquaviva C., Mamessier E. Revisiting the concept of stress in the prognosis of solid tumors: A role for stress granules proteins? Cancers. 2020;12:2470. doi: 10.3390/cancers12092470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Asadi M.R., Rahmanpour D., Moslehian M.S., Sabaie H., Hassani M., Ghafouri-Fard S., Taheri M., Rezazadeh M. Stress granules involved in formation, progression and metastasis of cancer: A scoping review. Front. Cell. Dev. Biol. 2021;9:745394. doi: 10.3389/fcell.2021.745394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sidali A., Teotia V., Solaiman N.S., Bashir N., Kanagaraj R., Murphy J.J., Surendranath K. AU-rich element RNA binding proteins: At the crossroads of post-Transcriptional regulation and genome integrity. Int. J. Mol. Sci. 2021;23:96. doi: 10.3390/ijms23010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lloyd R.E. Nuclear proteins hijacked by mammalian cytoplasmic plus strand RNA viruses. Virology. 2015;479–480:457–474. doi: 10.1016/j.virol.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McCormick C., Khaperskyy D.A. Translation inhibition and stress granules in the antiviral immune response. Nat. Rev. Immunol. 2017;17:647–660. doi: 10.1038/nri.2017.63. [DOI] [PubMed] [Google Scholar]
- 138.Le Sage V., Cinti A., McCarthy S., Amorim R., Rao S., Daino G.L., Tramontano E., Branch D.R., Mouland A.J. Ebola virus VP35 blocks stress granule assembly. Virology. 2017;502:73–83. doi: 10.1016/j.virol.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 139.Sun Y., Dong L., Yu S., Wang X., Zheng H., Zhang P., Meng C., Zhan Y., Tan L., Song C., et al. Newcastle disease virus induces stable formation of bona fide stress granules to facilitate viral replication through manipulating host protein translation. FASEB J. 2017;31:1337–1353. doi: 10.1096/fj.201600980R. [DOI] [PubMed] [Google Scholar]
- 140.Wang X., Wang H., Li Y., Jin Y., Chu Y., Su A., Wu Z. TIA-1 and TIAR interact with 5’-UTR of enterovirus 71 genome and facilitate viral replication. Biochem. Biophys. Res. Commun. 2015;466:254–259. doi: 10.1016/j.bbrc.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 141.White J.P., Cardenas A.M., Marissen W.E., Lloyd R.E. Inhibition of cytoplasmic mRNA stress granule formation by a viral proteinase. Cell Host Microbe. 2007;2:295–305. doi: 10.1016/j.chom.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 142.Soto-Rifo R., Valiente-Echeverria F., Rubilar P.S., Garcia-de-Gracia F., Ricci E.P., Limousin T., Décimo D., Mouland A.J., Ohlmann T. HIV-2 genomic RNA accumulates in stress granules in the absence of active translation. Nucleic Acids Res. 2014;42:12861–12875. doi: 10.1093/nar/gku1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Valiente-Echeverría F., Melnychuk L., Vyboh K., Ajamian L., Gallouzi I.E., Bernard N., Mouland A.J. eEF2 and Ras-GAP SH3 domain-binding protein (G3BP1) modulate stress granule assembly during HIV-1 infection. Nat. Commun. 2014;5:4819. doi: 10.1038/ncomms5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhou Y., Fang L., Wang D., Cai K., Chen H., Xiao S. Porcine reproductive and respiratory syndrome virus infection induces stress granule formation depending on protein kinase R-like endoplasmic reticulum kinase (PERK) in MARC-145 cells. Front. Cell. Infect. Microbiol. 2017;7:111. doi: 10.3389/fcimb.2017.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Albornoz A., Carletti T., Corazza G., Marcello A. The stress granule component TIA-1 binds tick-borne encephalitis virus RNA and is recruited to perinuclear sites of viral replication to inhibit viral translation. J. Virol. 2014;88:6611–6622. doi: 10.1128/JVI.03736-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bonenfant G., Williams N., Netzband R., Schwarz M.C., Evans M.J., Pager C.T. Zika virus subverts stress granules to promote and restrict viral gene expression. J. Virol. 2019;93:e0052019. doi: 10.1128/JVI.00520-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.López-Urrutia E., Valdés J., Bonilla-Moreno R., Martínez-Salazar M., Martínez-Garcia M., Berumen J., Villegas-Sepúlveda N. A few nucleotide polymorphisms are sufficient to recruit nuclear factors differentially to the intron 1 of HPV-16 intratypic variants. Virus Res. 2012;166:43–53. doi: 10.1016/j.virusres.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 148.Dinh P.X., Beura L.K., Das P.B., Panda D., Das A., Pattnaik A.K. Induction of stress granule-like structures in vesicular stomatitis virus-infected cells. J. Virol. 2013;87:372–383. doi: 10.1128/JVI.02305-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Catanzaro N., Meng X.J. Porcine reproductive and respiratory syndrome virus (PRRSV)-induced stress granules are associated with viral replication complexes and suppression of host translation. Virus Res. 2019;265:47–56. doi: 10.1016/j.virusres.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 150.Álvarez E., Castelló A., Carrasco L., Izquierdo J.M. Poliovirus 2A protease triggers a selective nucleo-cytoplasmic redistribution of splicing factors to regulate alternative pre-mRNA splicing. PLoS ONE. 2013;8:e73723. doi: 10.1371/journal.pone.0073723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Choi E.Y., Pintel D. Splicing of the large intron present in the nonstructural gene of minute virus of mice is governed by TIA-1/TIAR binding downstream of the nonconsensus donor. J. Virol. 2009;83:6306–6311. doi: 10.1128/JVI.00213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Katsanou V., Papadaki O., Milatos S., Blackshear P.J., Anderson P., Kollias G., Kontoyiannis D.L. HuR as a negative posttranscriptional modulator in inflammation. Mol. Cell. 2005;19:777–789. doi: 10.1016/j.molcel.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 153.Akira S., Maeda K. Control of RNA stability in immunity. Annu. Rev. Immunol. 2021;39:481–509. doi: 10.1146/annurev-immunol-101819-075147. [DOI] [PubMed] [Google Scholar]
- 154.Suswam E.A., Nabors L.B., Huang Y., Yang X., King P.H. IL-1beta induces stabilization of IL-8 mRNA in malignant breast cancer cells via the 3’ untranslated region: Involvement of divergent RNA-binding factors HuR, KSRP and TIAR. Int. J. Cancer. 2005;113:911–919. doi: 10.1002/ijc.20675. [DOI] [PubMed] [Google Scholar]
- 155.Jimenez-Boj E., Kedersha N., Tohidast-Akrad M., Karlhofer F.M., Stummvoll G., Zimmermann C., Ulrich W., Guiducci S., Hoefler E., Aringer M., et al. Autoantibodies to the translational suppressors T cell intracytoplasmic antigen 1 and T cell intracytoplasmic antigen 1-related protein in patients with rheumatic diseases: Increased prevalence in systemic lupus erythematosus and systemic sclerosis and correlation with clinical features. Arthritis Rheum. 2008;58:1226–1236. doi: 10.1002/art.23435. [DOI] [PubMed] [Google Scholar]
- 156.Ravi A., Chowdhury S., Dijkhuis A., Bonta P.I., Sterk P.J., Lutter R. Neutrophilic inflammation in asthma and defective epithelial translational control. Eur. Respir. J. 2019;54:1900547. doi: 10.1183/13993003.00547-2019. [DOI] [PubMed] [Google Scholar]
- 157.Navratilova Z., Novosadova E., Hagemann-Jensen M., Kullberg S., Kolek V., Grunewald J., Petrek M. Expression profile of six RNA-binding proteins in pulmonary sarcoidosis. PLoS ONE. 2016;11:e0161669. doi: 10.1371/journal.pone.0161669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yamaji M., Tanaka T., Shigeta M., Chuma S., Saga Y., Saitou M. Functional reconstruction of NANOS3 expression in the germ cell lineage by a novel transgenic reporter reveals distinct subcellular localizations of NANOS3. Reproduction. 2010;139:381–393. doi: 10.1530/REP-09-0373. [DOI] [PubMed] [Google Scholar]
- 159.Huelgas-Morales G., Silva-García C.G., Salinas L.S., Greenstein D., Navarro R.E. The stress granule RNA-binding protein TIAR-1 protects female germ cells from heat shock in Caenorhabditis elegans. G3 Genes Genomes Genet. 2016;6:1031–1047. doi: 10.1534/g3.115.026815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen J., Wang J., Qian J., Bao M., Zhang X., Huang Z. MBNL1 suppressed cancer metastatic of skin squamous cell carcinoma via by TIAL1/MYOD1/caspase-9/3 signaling pathways. Technol. Cancer Res. Treat. 2021;20 doi: 10.1177/1533033820960755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tian K., Qi W., Yan Q., Lv M., Song D. Signature constructed by glycolysis-immune-related genes can predict the prognosis of osteosarcoma patients. Investig. New Drugs. 2022;40:818–830. doi: 10.1007/s10637-022-01228-4. [DOI] [PubMed] [Google Scholar]
- 162.Wen J., Toomer K.H., Chen Z., Cai X. Genome-wide analysis of alternative transcripts in human breast cancer. Breast Cancer Res. Treat. 2015;151:295–307. doi: 10.1007/s10549-015-3395-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gottschald O.R., Malec V., Krasteva G., Hasan D., Kamlah F., Herold S., Rose F., Seeger W., Hänze J. TIAR and TIA-1 mRNA-binding proteins co-aggregate under conditions of rapid oxygen decline and extreme hypoxia and suppress the HIF-1α pathway. J. Mol. Cell. Biol. 2010;2:345–356. doi: 10.1093/jmcb/mjq032. [DOI] [PubMed] [Google Scholar]
- 164.Podszywalow-Bartnicka P., Wolczyk M., Kusio-Kobialka M., Wolanin K., Skowronek K., Nieborowska-Skorska M., Dasgupta Y., Skorski T., Piwocka K. Downregulation of BRCA1 protein in BCR-ABL1 leukemia cells depends on stress-triggered TIAR-mediated suppression of translation. Cell Cycle. 2014;13:3727–3741. doi: 10.4161/15384101.2014.965013. [DOI] [PubMed] [Google Scholar]
- 165.Abe Y., Mukudai Y., Kurihara M., Houri A., Chikuda J., Yaso A., Kato K., Shimane T., Shirota T. Tumor protein D52 is upregulated in oral squamous carcinoma cells under hypoxia in a hypoxia-inducible-factor-independent manner and is involved in cell death resistance. Cell Biosci. 2021;11:122. doi: 10.1186/s13578-021-00634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ravanidis S., Kattan F.G., Doxakis E. Unraveling the pathways to neuronal homeostasis and disease: Mechanistic insights into the role of RNA-binding proteins and associated factors. Int. J. Mol. Sci. 2018;19:2280. doi: 10.3390/ijms19082280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Barron V.A., Zhu H., Hinman M.N., Ladd A.N., Lou H. The neurofibromatosis type I pre-mRNA is a novel target of CELF protein-mediated splicing regulation. Nucleic Acids Res. 2010;38:253–264. doi: 10.1093/nar/gkp766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Barron V.A., Lou H. Alternative splicing of the neurofibromatosis type I pre-mRNA. Biosci. Rep. 2012;32:131–138. doi: 10.1042/BSR20110060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fleming V.A., Geng C., Ladd A.N., Lou H. Alternative splicing of the neurofibromatosis type 1 pre-mRNA is regulated by the muscleblind-like proteins and the CUG-BP and ELAV-like factors. BMC Mol. Biol. 2012;13:35. doi: 10.1186/1471-2199-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vanderweyde T., Yu H., Varnum M., Liu-Yesucevitz L., Citro A., Ikezu T., Duff K., Wolozin B. Contrasting pathology of stress granule proteins TIA-1 and G3BP in tauopathies. J. Neurosci. 2012;32:8270–8283. doi: 10.1523/JNEUROSCI.1592-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vanderweyde T., Youmans K., Liu-Yesucevitz L., Wolozin B. Role of stress granules and RNA-binding proteins in neurodegeneration: A mini-review. Gerontology. 2013;59:524–533. doi: 10.1159/000354170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jin K., Li W., Nagayama T., He X., Sinor A.D., Chang J., Mao X., Graham S.H., Simon R.P., Greenberg D.A. Expression of the RNA-binding protein TIAR is increased in neurons after ischemic cerebral injury. J. Neurosci. Res. 2000;59:767–774. doi: 10.1002/(SICI)1097-4547(20000315)59:6<767::AID-JNR9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]