Abstract
Among abiotic stresses, heat stress is described as one of the major limiting factors of crop growth worldwide, as high temperatures elicit a series of physiological, molecular, and biochemical cascade events that ultimately result in reduced crop yield. There is growing interest among researchers in the use of beneficial microorganisms. Intricate and highly complex interactions between plants and microbes result in the alleviation of heat stress. Plant–microbe interactions are mediated by the production of phytohormones, siderophores, gene expression, osmolytes, and volatile compounds in plants. Their interaction improves antioxidant activity and accumulation of compatible osmolytes such as proline, glycine betaine, soluble sugar, and trehalose, and enriches the nutrient status of stressed plants. Therefore, this review aims to discuss the heat response of plants and to understand the mechanisms of microbe-mediated stress alleviation on a physio-molecular basis. This review indicates that microbes have a great potential to enhance the protection of plants from heat stress and enhance plant growth and yield. Owing to the metabolic diversity of microorganisms, they can be useful in mitigating heat stress in crop plants. In this regard, microorganisms do not present new threats to ecological systems. Overall, it is expected that continued research on microbe-mediated heat stress tolerance in plants will enable this technology to be used as an ecofriendly tool for sustainable agronomy.
Keywords: heat stress, bio stimulant, microbes
1. Introduction
Heat stress is defined as the rise in the temperature of 10–15 °C above ambient. Heat stress negatively affects plant growth and development at all stages, from germination to harvesting [1,2,3]. Plants are sessile in nature and are exposed to variable temperature ranges. The optimal temperature for plant growth is 60–75 °F [4,5]. A high temperature is an environmental hazard and leads to abiotic stress that limits crop yield. Among all abiotic stresses such as drought, salinity, heavy metal exposure, and temperature, heat stress has the most devastating effect on plant metabolism and growth. Temperatures greater than 75 °F are referred to cause heat stress. Above the normal temperature range, plants restrict growth, development, and physiological cellular metabolism. Heat stress raises the morbidity and mortality of plants and deteriorate their quality [6,7,8]. If the duration of heat stress increases, it may cause irreversible changes, such as cellular destruction, in plant cells. Plants show various signs of heat stress, such as wilting, leaf damage, fruit drop, blossom end rot, and bolting [9,10].
Higher temperatures lead to a cascade of cellular functions and the release of heat shock proteins (HSPs), which minimize cellular damage in plants. Heat stress affects the physiological processes of plant growth and development in several ways [11,12]. Several studies conducted worldwide suggest various regulators of heat stress using different omics approaches, as shown in Table 1. Heat stress increases membrane fluidity, leading to the uncoupling of a reaction series resulting in altered metabolism, and impairs cell machinery and chromatin changes in plants. The uncoupling of reactions leads to accretion of intermediate products and reactive oxygen species in plant cells. Heat stress that turns the central dogma blocks the degradation of proteins and disturbs the cytoskeleton of plant cells [13,14,15]. The thylakoid membrane of chloroplasts falls off in response to heat stress, which minimizes the function of the electron transport chain and impairs photosynthesis in the photosystem II (PSII) [16]. A comparative analysis of the gradual heat stress response and shock heat stress response was conducted in strawberry plants [17]. The results showed a high level of peroxidase and minimal protein content. Increased peroxidase activity is involved in thermotolerance [17]. Triticum aestivum subjected to heat stress restricted plant seedling characteristics and germination index. The plant produced reactive oxygen species and antioxidant enzymes that impaired photosynthesis and degraded proteins, thereby affecting the entire germination process [18].
Table 1.
Physiological and molecular responses in plants against heat stress (NA: Not Applicable).
| References Country Year |
Plants | Model/Approach | Heat Stress Regulators |
|---|---|---|---|
| Kotak et al. [22] 2007 |
Arabidopsis | Omics | Phytohormone HS MBF1c HOT2 |
| Postgate et al. [36] 2013 |
Arabidopsis | Microarray | HSP70 HSP60 APX |
| Peoples et al. [37] 2007 |
NA | Appraisal | TATA box proximal 5′ flanking regions |
| Allahverdiyeva et al. [38] 2004 |
Arabidopsis, Lycopersicon esculentum | Experimental | HsfA1,2 HsfB1 |
| Giller et al. [39] 2001 |
Lycopersicon esculentum, Citrullus lanatus | Experimental | Phenolic components |
| Szymanska et al. [12] 2011 |
NA | Appraisal |
Dhn, Sag, Sgr |
| Ghosh et al. [17] 2004 |
Fragaria × ananassa | Experimental | Antioxidant enzymes |
| Saha et al. [18] 2010 |
Triticum aestivum | Experimental | Cellular, molecular and metabolic cascade |
| Glick et al. [40] 1999 |
Soybean and Arabidopsis | In vivo and In vitro | HSP90 HSP60 HSP20 |
Heat stress is an extremely serious issue that is responsible for extensive crop loss and will likely worsen in the future [19,20,21]. Temperatures above the optimal threshold value have a negative impact on crop physiology from mild to permanent damage. Since heat stress is a direct consequence of climate change, which ultimately increases the frequency of heatwaves, resulting in global warming [22], ensuring plant recovery and survival becomes a major challenge [23,24,25]. Moreover, as global warming worsens daily, strategies to enhance plant thermotolerance are urgently needed.
Various measures can be taken to minimize heat stress such as shading and deep-water planting of vulnerable plants. Furthermore, microbes are fundamental living components on Earth that provide sustainability to plants against various stresses and provide nutrition and resistance to combat diseases [26,27,28]. Microbial application is an advanced, globally accepted, environmentally friendly, and sustainable technique that uses soil microbes in stress-compromised plants to lessen the lethal effects of ecological stress. It is cheaper, ecofriendly, and easily available; it can be adopted and applied to produce high-quality yields. Plant–microbial interactions enhance the accessibility of plants towards organic materials [29]. They also play an important role in sustainable agronomy and ecology. Plant growth-promoting rhizobacteria synergistically improve plant growth by producing phytohormones, minimizing stress levels, and elevating the nutritional status [30,31]. Cyclic phosphorylation induces the expression of HSPs, which are molecular chaperones that may also be produced by galactinol synthases [13,32]. Beneficial microorganisms associated with plants can improve their resistance towards biotic and abiotic stresses. They alleviate adverse effects of stress and promote plant growth [33,34,35]. It is important to explore the plant microbial community that contributes towards providing resistance against different environmental stresses. Only a few studies have reported plant–microbial interactions in tackling heat stress in plants. The aim of this study was to gather information regarding plant microbial endophytic and rhizospheric communities in the mitigation of heat stress. Such information may enhance our current understanding of the role of microorganisms in plant stress mitigation and thus enabling their use in a strategy to attain sustainable agriculture under changing climatic conditions.
2. Role of Microorganisms in Thermotolerance
Microbes are biological control agents that combat heat stress. Microbial inoculation causes thermotolerance. Exopolysaccharides are released by bacteria under heat stress containing 97% water, which improve the soil structure. Water remains available to plants, so it is helpful during the stress period. Thermotolerance is a complex mechanism. It has also been hypothesized that the production of proline and glycine betaine contributes to thermoregulation [41,42]. The details of the two major categories of thermotolerant microbes are discussed as below.
2.1. Endophytes
Endophytes are microorganisms that live in plant cells and form biofilms that interact with plant exudates. Endophytes are used as biostimulants to produce various compounds. Most endophytes are inaccessible because they live inside plant tissues and remain in symbiotic relationships [43,44]. In the light of plant–microbe interaction-mediated heat stress mitigation, only limited studies are available. Endophytes form symbiotic relationships with plants. To maintain a stable relationship, they produce various kinds of compounds that promote the growth and development of the plants. They produce biochemicals that cannot be synthesized [45,46]. Evidence of endophytes, their mode of action, and their growth-promoting traits have been reported by increasing number of recent publications, indicating their importance.
A field experimental study was designed to evaluate the inoculation effects of endophytic microbe SA187 on Arabidopsis thaliana and wheat plants [47]. The plants were divided into two groups: the untreated normal group, and the group inoculated with Enterobacter SA187. The plants were exposed to high temperatures up to 44 °C to induce heat stress. Agronomic traits were also assessed. Enterobacter sp. SA187 induced thermotolerance in plants and promoted thermopriming. The results were repeated over three consecutive seasons. The inoculation treatment group showed an increase in overall plant biomass and height by 10–14%, grain yield by 40%, and seed weight by 12%. These results suggest that SA187 inoculation is beneficial to plants to enhance the heat tolerance [47].
Meena et al. studied tomato plant seedlings subjected to heat and drought stress. Septoglomus deserticola and S. constrictu were inoculated, and cellular parameters were measured. Inoculation decreased oxidative stress and minimized the level of reactive oxygen species. The symbiotic effect improved cellular performance, stomatal conductance, and leaf water content. The mycorrhizal inoculation improved and enhanced physiological features under combined stress [48].
The inoculation of B. cereus SA1 on soybean plants under heat stress conditions causes thermoregulation [49]. The analysis showed increased chlorophyll a and b, carotenoid, protein, ascorbic acid peroxidase, and superoxide dismutase levels in plants. SA1 significantly improved thermoregulation [49] (Table 2).
Table 2.
Application of endophytes in mitigating heat stress in plants (↑: Increase Traits, ↓: Decrease Traits).
| References Country Year |
Microbes | Model | Plant | Parameters | MOA | Stress | Effect |
|---|---|---|---|---|---|---|---|
| Park et al. [47] 2021 |
Enterobacter SA187 | Vitro Experimental field |
Arabidopsis thaliana, Wheat plant |
↑ Biomass, ↑ 10–14% height,↑ 40% grain yield and seed weight 12%. | Chromatin modification | Long term | Beneficial |
| Meena et al. [48] 2018 |
Septoglomus deserticola and Septoglomus constrictu |
In vitro | Solanum lycopersicum | Improved Stomal conductance, water content and leaf water content | ↓ Oxidative stress | Heat +drought | Improved |
| Anli et al. [41] 2011 |
Pseudomonas fluorescens, Pantoea agglomerans, Mycobacterium sp., Bacillus amyloliquefaciens, Pantoea agglomerans | Appraisail | Triticum aestivum | HSP90 Antioxidant enzymes, HSTP |
Thermoregulation | High temp | Significant |
| Anli et al. [41] 2011 |
B. phytofirmans | NA | Solanum tuberosum | ↑ Proline and glycine betaine | Thermotolerance | High temperature | Good biocontrol |
| Bisht et al. [49] 2020 |
B. cereus SA1 | Experimental | Soybean | ↑ Chlorophyll a and b ↑ Carotenoid, ↑ Chlorophyll florescence |
Thermotolerant | Medium to high temp | Bio fertilizer |
2.2. Plant Growth-Promoting Rhizobacteria (PGPR)
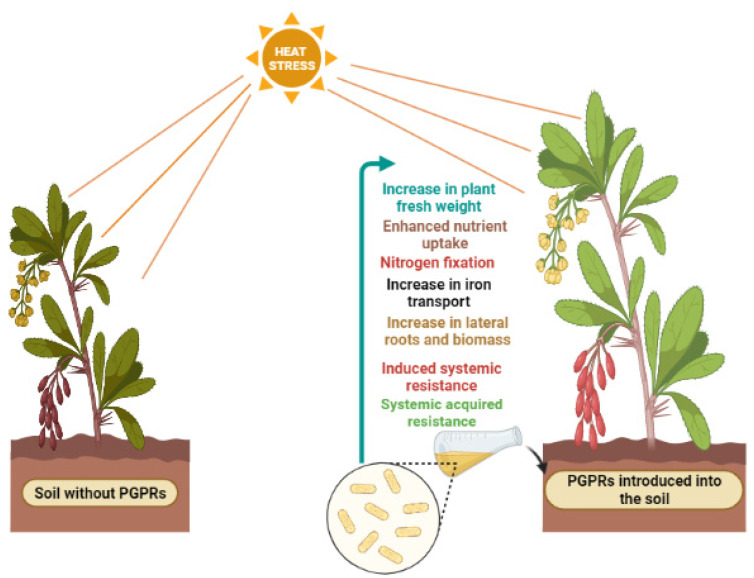
Bacteria that colonize in the roots of plants or along the rhizopheric axis and promote plant growth are known as PGPR. They promote plant growth directly by regulating nutritional status (phosphate solubilization, N fixation, iron sequestration) and hormone synthesis (IAA, GB, CK, etc.). They also promote plant growth indirectly by providing immunity to plants against environmental stress and by producing compatible solutes such as proline, sugars, organic acids, and glycine betaine [50,51]. Table 3 summarizes the recent studies on inoculation of PGPR to plants under heat stress, their effects, and mechanism of action. Overall, the inoculation of PGPR is beneficial to the plants to overcome the deleterious effects of heat stress as illustrated in Figure 1.
Table 3.
Application of plant growth-promoting rhizobacteria in mitigating heat stress (↑: Increase Traits, ↓: Decrease Traits).
| References Country Year |
Microbes | Model | Plant | Parameters | MOA | Stress | Effect |
|---|---|---|---|---|---|---|---|
| Abd El-Daim et al. [57] 2014 |
Bacillus amyloliquefaciens UCMB5113 | Field | Triticum aestivum | ↑ Survival * rate | ↓ GR ↓ APX ↓ HPS17 |
Short | Beneficial |
| Rana et al. [54] 2012 |
Curvularia proturberata isolate Cp4666D, Burkholderia phytofirmans PsJN |
Field experiment |
Triticum aestivum, Dichanthelium lanuginosum, Solanum lycopersicum |
Production of IAA, cytokines, protein and ↑ chlorophyll | ↓ Pathogen, ↓ ROS | Heat stress | Beneficial |
| Mitra et al. [59] 2021 |
Bacillus cereus, Pseudomonas, Serratia liquefaciens, P. fluorescens and Pseudomonas putida |
In vitro |
S. lycopersicum L., Cajanus cajan, G. max, and Triticum spp. |
↑ ACC-deaminase, Production, ↑ phytohormone and ↑ antioxidant defense |
Thermal tolerance | High temp | Sustainable |
| Maitra we al. [58] 2011 |
Aeromonas hydrophilla
Serratia liquefaciens Serratia proteamaculans |
In vitro | Glycine max | ↑ Exopolysacchrides production | Thermotolerance | High temp | Remarkable |
| Kang et al. [53] 2019 |
Bacillus tequilensis SSB07 | Experimental | Glycine max | ↑ Gibberellins ↑ IAA and ↑ ABA, jasmonic acid and salicylicacid contents |
Thermotolerant | Moderate | Improvement |
| Ali et al. [55] 2011 |
Pseudomonas putidaAKMP7 | Experimental | Triticum aestivum | ↑ Root and shoot length, ↑ biomass, ↑ SOD, ↑ CAT and APX | Thermotolerant | High | Improvement |
| Ali et al. [56] 2009 |
Pseudomonas AKM-P6 | Experimental | Sorghum | ↑ Cellular metabolites | Thermotolerant | High | Improvement |
| Park et al. [47] 2017 |
Bacillus aryabhattai SRB02 | Experimental | Glycine max | ↑ ABA ↑ IAA, JA, GAs contents, | Fertilizers+ thermotolerance | Medium | Improvement |
| Meena et al. [48] 2015 |
Pseudomonas aeruginosa 2CpS1 | Net house experiment | Triticum aestivum cultivar (HUW-234) | ↑ Plant height and root length, ↑ chlorophyll content, | Mitigation | High | Improvement |
| 2018 | Bacillus amyloliquefaciens | Experimental | Oryza sativa | ↑ Proline, Total Soluble Sugar, ↑ Lipid Peroxidation and over expression of six stress-responsive of dehydrin (DHN), glutathione S- protein 6 (NRAMP6) genes |
↑ Modulated stress-responsive gene expressions ↑ phytohormone |
High | Significant |
| Issa et al. [52] 2018 |
Paraburkholderia phytofirmans PsJN |
Green house experiment | Lycopersicon esculentum | ↑ Growth Biomass Chlorophyll content |
↑ Chlorophyll content, Photosystem II, ↑ Accumulations of sugars, total amino acids, proline, and Malate. |
Thermotolerant | Improvement |
Figure 1.
Metabolic reprogramming of the plant cell under heat stress.
Microbes are beneficial for the thermotolerance of plants. In the field experiments, Triticum aestivum was selected as a model plant for inoculation with Bacillus amyloliquefaciens UCMB5113 and was exposed to short-term heat stress. Glutathione reductase and transcription factors were selected as gold standards for comparative analysis. The results showed that the inoculated plants had reduced APX1, GR, SAMS1, and HSP17 expression. The recovery and survival of inoculated plants were more significant than those of non-inoculated plants [52]. The inoculation of B. cereus SA1 on soybean plants under heat stress conditions caused thermoregulation. The analysis showed increased chlorophyll a and b, carotenoid, protein, stress tolerant enzymes levels in plants. SA1 significantly improved thermoregulation [49].
In 2021, in vitro experimental plants grown in a growth chamber suggested that PGPR play a key role in thermotolerance. The bacterial species Bacillus cereus, Pseudomonas spp., Serratia liquefaciens, P. fluorescens, and Pseudomonas putida, which were hosted in Solanum lycopersicum L, Cajanus cajan, G. max, and Triticum spp., caused the production of phytohormones, antioxidant enzymes, and ACC-deaminase consequently mitigated heat stress [53].
The microbial isolates Burkholderia phytofirmans PsJN, Curvularia proturberata isolate Cp4666D, which were hosted in T. aestivum, Dichanthelium lanuginosum, and S. lycopersicum caused remarkable thermotolerance in plants. Microbes increased the production of IAA and cytokines, as well as the molecular protein and chlorophyll contents, suppressing plant pathogens and production of free radicals [54].
Kang et al. conducted an experimental study in the Republic of South Korea on Glycine max (Soybeans) that was subjected to heat stress to reveal the effect of microbial inoculation. The heat stress conditions were a day/night cycle of 16 h at 38 °C and 8 h at 30 °C for 1 week. Soybeans are highly sensitive to heat stress, and the use of PGPR counters the negative effects of heat stress. The bacterial strain Bacillus tequilensis SSB07 was inoculated into the plant and various growth attributes such as seedling growth and production of GBs, IAA, and ABA were recorded. SSB07 increased shoot length and biomass. SSB07 countered the negative effects of heat stress on crop growth and development [55].
The Pseudomonas sp. strain AKM-P6 was inoculated into sorghum. Sterilized seeds were smeared with a talc-based formulation (108 cells/g) of strain AKM-P6 and sown in plastic cups. Five-day-old seedlings were exposed to heat stress and harvested after 10 d. The samples were analyzed using a scanning electron microscope, and other plant biochemical parameters were assessed. The conditions were a temperature of 47–50 °C during the day and 30–33 °C at night for 10 days. The microbial strain AKMP6 improved the high temperature stress in sorghum seedlings. The microbial strain AKMP6 helped sorghum seedlings endure and grow at preeminent temperatures for up to 15 days, whereas the inoculated plants died after 5 days. Bacterial inoculation promoted the biosynthesis of high-molecular-weight proteins in leaves under increased temperature, compact membrane injury, and increase in the levels of cellular metabolites. The strain AKM-P6 augmented the lenience of sorghum seedlings by inducing physiological and biochemical changes in the plants [56].
Triticum aestivum was inoculated with Pseudomonas putida strain AKMP7. The disinfected seeds were planted in plastic pots. After 2 weeks, each seedling was dispersed in one pot. The plants were unprotected from heat stress, and after 95 d, the seedlings were collected to assess their growth and enzymatic activities on the 110th day of growth. The temperature conditions were 37–40 °C during the day and 27–30 °C at night for 95 d. The inoculation of AKMP7 increased the levels of cellular constituents, plant development, and total biomass. AKMP7 also convalesced with the survival and growth of wheat plants under heat stress by increasing their root and shoot length, dry biomass, tiller, spike, grain formation, and reducing membrane injury and antioxidant enzyme activities such as SOD, APX, and CAT activities under heat stress. The results showed that AKMP7 could be effective in relieving heat stress and subsequently improving the growth of wheat plants under heat stress [55]. In other trials, the thermotolerance potential of Glycine max inoculated with Bacillus aryabhattai SRB02 and subjected to 38 °C/30 °C day/night heat stress for 0, 12, and 48 h was measured. At vegetative stage 3 (V3), 10 mL of bacterial culture (1 × 108 cfu/mL) was applied for 3 days. Growth parameters were recorded after the application of heat stress. SRB02-treated soybean plants showed significantly better thermotolerance than the untreated plants, based on their ABA-mediated stomatal closure and increased IAA, JA, and GA contents, plant growth, and biomass. SRB02 also endured extraordinary nitrosative stress induced by the nitric oxide donors GSNO and CysNO. These results suggest that SRB02 may be a valuable source of biofertilizers to increase crop production [47].
In 2014, Abd El-Daim performed an experimental study on microbial mitigation of heat stress in wheat crops. Triticum aestivum seeds were soaked in a bacterial suspension (1 × 107 cfu/mL) for 2 h at 28 °C and grown in pots in a growth chamber for 12 days. The plants were subjected to a higher temperature of 45 °C, following which the expression levels of ascorbate peroxidase (APX1), S-adenosylmethionine synthetase (SAMS1), HSP17.8, heat-inducible transcription factor (HsfB1), heat shock factor 3 (HsfA3), and MBF1c was determined. Bacterial treatment improved the heat stress control in both cultivars of wheat by levering the transcription levels of several stress-related genes and ascorbate-glutathione enzymes. Seeds treated with two PGPR strains that colonized the roots amended their thermotolerance [57].
In 2015, another scientist, Meena, conducted an experiment using another bacterial species to observe the effect of beneficial microbes under heat stress in plants. A T. aestivum cultivar (HUW-234) was inoculated with Pseudomonas aeruginosa strain 2CpS1 in a greenhouse experiment. The growth chamber conditions were 30 °C/25 °C day/night for 24 days. Strain 2CpS1 increased the seedling length, leaf area, biomass, total chlorophyll content, relative water content, and soil moisture contents. The application of stress-tolerant PGPR strains could be used as a reasonable approach for cultivating crops at elevated temperatures [48].
In another experiment, T. aestivum were subjected to high temperature stress, and their thermotolerance potential towards heat stress was recorded. Seeds were grown in hydroponics for 7 days, and after one day of microbial constrain of SN13 inoculation, seedlings were exposed to 45 °C. After completing the experiment, physiological and biochemical factors such as membrane potential, osmolytes accumulation, proline content, lipid peroxidation, total soluble sugar, and six stress-responsive genes were assessed. The results suggest that SN13 positively controls the expression of stress-responsive genes and phytohormones, suggesting its multidimensional role in stress response. The differential responses of rice seedlings to heat stress and phytohormones were confirmed using principal component analysis (PCA) based on the effects of SN13 inoculation on the response of rice to heat stress and phytohormone treatments [58].
Moreover, 23-day-old sprouts of Lycopersicon esculentum were bio-primed with a bacterial inoculum of Paraburkholderia phytofirmans strain PsJN at 106 CFU/mL and planted into the green house. Leaf gas exchange ratio, chlorophyll fluorescence rate, photosynthetic pigment content, and other parameters were evaluated. The greenhouse temperature was maintained at 32 °C under 16 h of light and at 27 °C for 8 h of dark for 45 days. PsJN improved plant growth attributes such as chlorophyll content, photosystem II, and sugar and total protein content. The PsJN strain can improve the destructive effects of heat stress by stimulating the thermotolerance mechanism of tomato plants [52].
3. Physiological Changes Induced by Thermotolerant Microbes in Plants under Heat Stress
3.1. Photosynthesis
Photosynthesis is a natural cellular respiration process by which plants convert light energy into chemical energy [60]. Heat stress disrupts the photosynthetic apparatus, resulting in the inhibition of plant growth and development. Studies have suggested that it inhibits the production of ribulose 1, 5-bisphosphate (RuBP), which is involved in the electron transport chains [61,62]. Heat stress also inactivates enzymes involved in photosystem 11 lowering the rate of photosynthesis [63,64]. However, under heat stress, oxygenic microbes contain light-harvesting pigments that induce the reprogramming of cellular events in the thylakoid membrane [65,66,67]. They release oxygen and absorb carbon dioxide. Cyanobacteria are one of the bacteria that support photosynthesis and promote plant growth [68].
3.2. Changes in Respiration
Respiration is a chemical process that involves oxygen and glucose to produce energy for plant survival and is important in maintaining plant growth as well as the carbon cycle [69,70]. Higher temperatures enhance cellular respiration owing to the increased kinetic energy. However, most of this energy is apportioned to maintain respiration, resulting in a general reduction in the energy utilization efficiency of plants [70,71]. Beneficial microbes increase soil respiration and improve nutrient cycling in plants. Microbes can minimize stress levels and restore ecosystems to an equilibrium state. Plant–microbe interactions maintain nitrogen, hydrogen, sulfur, and oxygen levels in a biogeochemical cycle [72,73].
3.3. Stomatal Closure
Stomata are microscopic openings present in the epidermis of leaves. Stomatal opening and closing are important for maintaining the physiological functions such as transpiration, and stomatal closure is a common adaptative response to heat stress [74,75]. Under heat stress, there is a possibility of rapid water loss. Plant–microbial interaction enhances the production of abscisic acid (ABA), a phytohormone, also known as a stress hormone, that activates various biotic and abiotic stress conditions, causes the closure of stomata, and is also important in osmoregulation [76,77]. The microbial production of ABA causes simultaneous stomatal closure [78,79]. Plants close their stomata to reserve water loss caused by evaporation [75].
4. Molecular Mechanism of Action of Microbes in Mitigating Heat Stress in Plants
4.1. Nitrogen Fixation
Nitrogen fixation is a natural process by which gaseous N2 is converted into biological forms of NH3 and NH4. Nitrogen is a macronutrient in plants. Heat stress promotes N accumulation in the meristems of plant cells via apical blade erosion and plays a vital role in energy metabolism, protein synthesis, and photosynthesis [80,81]. Higher temperatures delay the development of nitrogenase activity in plants, resulting in inhibition of N fixation, leading to stunted growth of plants [82,83]. Microbes can mitigate heat stress by enhancing N fixation. Microbes can transfer inert atmospheric N into the most reactive forms of ammonia, nitrates, and nitrites through a series of chemical reactions [84,85,86]. Thus, microbes possess a relatively symbiont relationship with plant species known as diazotrophs [87,88]. There are two kinds of nitrogen fixating microbes (symbiotic and no symbiotic) that improve the soil nitrogen concentration, rhizobacterial population levels, soil nitrogenase activities and N uptake in plants [89]. Zhang et al. in [90] tested the beneficial effect of several bacterial strain on soybean growth under suboptimal temperature and found that the bacterial growth promoting effects are caused by the bacterial nitrogen fixing potential. Various recent studies are reported in favor of mitigation of heat stress by nitrogen fixing bacteria [91,92].
4.2. Microbial Production of Siderophore
Siderophores are organic compounds with low molecular weights. These compounds have a high affinity for iron-chelating compounds. Microbes can produce siderophores, which are microscopic, high-affinity iron-chelating combinations. These serve primarily to transport iron across the cell membranes through membrane receptors. Various gram-positive and gram-negative bacteria produce and secrete siderophore to scavenge iron from the environment [93,94]. Plant–microbial interactions enhance siderophore production, ultimately improving the nutritional status and growth of plants under stress [95]. Application of siderophore producing bacteria Pseudomonas putida and Pseudomonas sp. have shown the improvement in growth, chlorophyll and plant biomass in wheat and sorghum under heat stress [55,56].
4.3. Microbial Production of 1-Aminocyclopropane-1-carboxylate (ACC) Deaminase
Some microorganisms can produce the enzyme ACC-deaminase and promote plant growth by sequestering and splitting plant-produced ACC, producing α-ketobutyrate and ammonia, which lowers the level of ethylene in plants. This is the most efficient mechanism of action for plants to tolerate stress and promotes a much easier lifestyle in the soil [96,97,98]. Plant–microbial interactions enhance ACC-deaminase production, which facilitates plant growth under stress conditions [99]. Recently reported ACC deaminase activity produced Achromobacter piechaudii, which moderated ethylene metabolism and ultimately resulted in better heat tolerance in pepper [100]. Furthermore, ACC deaminase producing Brevibacterium linens enhance combined heat and UV-B radiation stress in rice plant and enhance plant biomass, photosynthetic traits and decrease ethylene emission [101]. In another study of Mukhtar et al. in [102], they reported that ACC deaminase producing Bacillus cereus mitigate heat stress in tomato and observed drastic morphological and physiological effects on tomato plants under heat stress.
4.4. Microbial Production of Phytohormone
In response to stress, microbes can produce phytohormones that act as endogenous growth regulators by reducing stress and optimizing plant growth. Microbes produce various hormones such as gibberellin (GB), cytokinin (CK), salicylic acid (SA), indole-3-acetic acid (IAA), and ABA [103,104]. The mechanism of stress mitigation involves modulating antioxidant potential and maintaining the osmolyte potential of plants. Most prominently, ABA is produced under stress conditions and is termed a stress hormone. It causes stomatal closure, preventing osmolyte loss through evaporation [57,105]. Phytohormones form signaling networks. Various studies have suggested that the exogenous application of ABA mitigates heat stress and its consequences. ABA is a vital hormone that reduces oxidative stress by activating the defense system, leading towards redox homeostasis [54,55,59]. Plant–microbial interaction also enhances the production of the GB hormone, which is important in regulation of developmental process such as germination, flowering, fruit, and leaf senescence. It controls major aspects of plant growth. Under plant–microbial interaction, there is an increase in the level of auxin, which positively modulates the genetic expression and enhances the activity of the defensive antioxidant system of the plants [106]. Auxin producing Azospirillum brasilense was reported to mitigate heat stress in wheat by maintaining water status [107]. Khan et al. had demonstrated how thermotolerant Bacillus cereus mitigate heat stress in tomato and soybean through moderation in the auxin levels. Similarly, gibberellins is another phytohormone that are produce by bacteria and are involve in all plant growth and development including stem elongation, leaf expansion and fruit ripening [108]. Atzorn et al. in [109] reported gibberellin producing bacteria first time in Rhizobium meliloti. Nowadays, several genera of Pseudomonas, Serratia, Bacillus, and Arthrobacter bacteria have been reported for the production of different GAs.
4.5. Molecular Approaches
To sustain crop yield through thermotolerance, plants evolve through a series of cascade events. Previous electronic data on heat stress phenomena are available, including data on the production of classical chaperone proteins. In Germany, an experiment was designed to evaluate the thermotolerance mechanism of Arabidopsis. Results showed that phytohormones (ABA, SA, and ethylene), oxidative stress, and several mutants were involved in thermotolerance. An applied molecular dynamics study revealed that the genes MULTIPROTEIN BRIDGING FACTOR 1c (MBF1c) and HOT2, which encode chitinase-like proteins, are involved in acquiring hemostatic heat tolerance [110].
Molecular dynamics studies have extended our knowledge of plant thermotolerance and its associated genes. Microarrays suggest that heat stress causes the development of various HSPs. Among them, HSP70, APX, and HSP60 are potent and thermodynamically involved [36]. The responses of plants to heat stress are unique. Various studies have strengthened our understanding of the vital roles of HSPs. HSP70, HSP90, and HSP20b function to analyze mutants, denature proteins, and for homooligomerization, respectively [40]. Heat stress deranges chromatin organization in plant cells. JUMONJI (JMJ proteins are responsible for chromatin organization. These are histone demethylase proteins found in nature [111,112,113]. Demethylases are enzymes that eliminate methyl groups from molecules and promote structural support to chromosomes. Plants maintain heat memory stress because of lowered H3K27me3 (histone H3 lysine 27 trimethylation) expression in small heat shock genes [114,115,116]. These are an important family of proteins that control heat shock genes, thus allow plant cells to tolerate heat stress via a memory mechanism [117,118,119].
The production and accumulation of HSPs range from molecular mass 10 kDa to 100 kDa. Studies have shown that the application of genetic stock, where heat shock elements are present in a TATA box in the proximal 5 flanking regions of heat shock genes, and the application of osmoprotectants can combat heat stress [37]. In 2011, a study on heat stress reported detailed information about the heat mechanism wherein senescence-associated genes (sag), dehydrins (dhn), and HSP stay-green (sgr) genes are involved in stabilizing heat stress. Plant thermotolerance can be improved through molecular breeding [12]. To minimize heat stress, more than 20 heat shock factors are involved in initiating a cascade event series to trigger, maintain, and recover the plants. Two main factors HsfA1, 2 and HsfB1 are responsible for thermotolerance. HsfB1 is a co-regulator that activates Hsf, a working horse that is activated in the summer [11]. Acute and chronic heat stress affects plant growth, development, and yield [120].
4.6. Microbial Production of Volatile Compounds
Plant–microbial interactions enhance the production of volatile compounds. Various microorganisms, such as Bacillus and Pseudomonas produce volatile compounds when inoculated into the host plants. These are important compounds with a range of more than 200 types such as isoprenoids and terpenoids, which are known as growth inducers. They also promote the defense system of plants. In an experimental setup of tomato and watermelon plants grown in a growth chamber for 30 days at various temperatures, the authors evaluated the responsive components against heat stress. Moreover. HSPs and other plant components are also involved in thermoregulation. The acclimatization of plants is because of the bioactivity of phenolic compounds and the inhibition of oxidation [39,121,122].
4.7. Organic Acid Production
Organic acid are considered as an essential source of carbon and are rich in vitality. Plant growth promoting bacteria synthesize various secondary metabolites such as phytohormones and organic osmolytes that activate host plants stress management mechanisms and solubilize nutrients for easy absorption by plants [123]. Organic acid producing bacteria alleviate heat stress and enhance plant growth in soybean and tomato [105,124,125]. Table 1 summarizes the physiological and molecular approaches of microbes in mitigation of heat stress.
5. Conclusions and Future Prospective
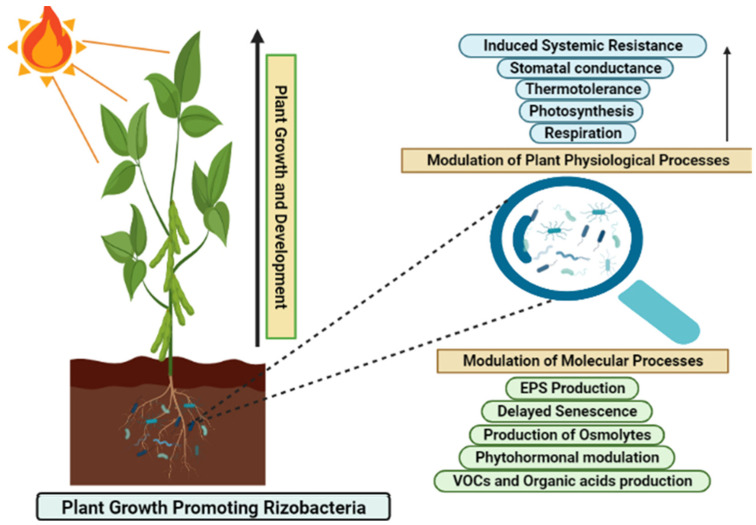
It is evident that there is an increasing demand for sustainable agronomy to meet the needs of the growing population and enhance agronomic yield without disturbing ecological components. Since 2000, there has been an increase in the demand for bio stimulants as growth promoters, and research interest and publication are increasing in the area of heat stress management using beneficial microbes [126,127]. Microorganism inoculation enhances resistance and tolerance towards heat stress. The isolation and identification of beneficial microbes in the cross-protection of plants would be highly valuable. Beneficial microorganisms alleviate the adverse effects of stress through the production of phytohormones and certain metabolites and enhance plant defense systems as shown in Figure 2 (Both images were created using Biorender.com). Plant–microbial interactions activate the antioxidant defense system. They have also been found to improve ion homeostasis by maintaining osmo-protectant levels. Therefore, it is important to explore plant-associated microbial communities. Only a few studies have reported plant–microbial interactions in heat stress regulation. Overall, this review suggests that use of microorganisms needs to be thoroughly explored in the field of agronomy for mitigating heat stress and attaining the goal of sustainable agriculture.
Figure 2.
The illustration represents the role of plant growth-promoting rhizobacteria in mitigating heat stress in plants. PGPRs promote plant growth and development by modulating the physiological and molecular processes in plants.
Acknowledgments
The authors wish to thank the Crop Physiology Lab and the Basic Science Research Program (National Research Foundation of Korea (NRF)) for their support.
Abbreviations
| IPCC | Intergovernmental Panel on Climate Change |
| HSPs | Heat shock proteins |
| DHN | Dehydrins |
| NRAMP6 | Natural resistance-associated macrophage protein 6 |
| PGPR | Plant growth-promoting rhizobacteria |
Author Contributions
Conceptualization, methodology, writing original draft: S.S., M.A.K. and M.I.; formal analysis investigation: S.-M.K., S.H.W. and A.P.; critical review editing, visualization, graphic designing: W.R., S.A.K., D.B. and E.-H.K.; formatting, resources and data curation, visualization, supervision, and funding acquisition: I.-J.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the present study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as potential conflict of interest.
Funding Statement
This research was funded by the “Basic Science Research Program” through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2020R1I1A1A01065443).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ali M.G., Ahmed M., Ibrahim M.M., El Baroudy A.A., Ali E.F., Shokr M.S., Aldosari A.A., Majrashi A., Kheir A. Optimizing sowing window, cultivar choice, and plant density to boost maize yield under RCP8. 5 climate scenario of CMIP5. Int. J. Biometeorol. 2022;66:971–985. doi: 10.1007/s00484-022-02253-x. [DOI] [PubMed] [Google Scholar]
- 2.Ejaz M., Abbas G., Fatima Z., Iqbal P., Raza M.A., Kheir A., Ahmed M., Kakar K.M., Ahmad S. Modelling Climate Uncertainty and Adaptations for Soybean-Based Cropping System. Int. J. Plant Prod. 2022;16:235–250. doi: 10.1007/s42106-022-00190-8. [DOI] [Google Scholar]
- 3.Ding Z., Ali E.F., Elmahdy A.M., Ragab K.E., Seleiman M.F., Kheir A.M. Modeling the combined impacts of deficit irrigation, rising temperature and compost application on wheat yield and water productivity. Agric. Water Manag. 2021;244:106626. doi: 10.1016/j.agwat.2020.106626. [DOI] [Google Scholar]
- 4.Marchin R.M., Backes D., Ossola A., Leishman M.R., Tjoelker M.G., Ellsworth D.S. Extreme heat increases stomatal conductance and drought-induced mortality risk in vulnerable plant species. Glob. Chang. Biol. 2022;28:1133–1146. doi: 10.1111/gcb.15976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee A., Roychoudhury A. Plant Metabolites and Regulation under Environmental Stress. Elsevier; Amsterdam, The Netherlands: 2018. Small heat shock proteins: Structural assembly and functional responses against heat stress in plants; pp. 367–376. [Google Scholar]
- 6.Dos Santos T.B., Ribas A.F., de Souza S.G.H., Budzinski I.G.F., Domingues D.S. Physiological Responses to Drought, Salinity, and Heat Stress in Plants: A Review. Stresses. 2022;2:113–135. doi: 10.3390/stresses2010009. [DOI] [Google Scholar]
- 7.Haider S., Raza A., Iqbal J., Shaukat M., Mahmood T. Analyzing the regulatory role of heat shock transcription factors in plant heat stress tolerance: A brief appraisal. Mol. Biol. Rep. 2022:1–15. doi: 10.1007/s11033-022-07190-x. [DOI] [PubMed] [Google Scholar]
- 8.Perrella G., Bäurle I., van Zanten M. Epigenetic regulation of thermomorphogenesis and heat stress tolerance. New Phytol. 2022;234:1144–1160. doi: 10.1111/nph.17970. [DOI] [PubMed] [Google Scholar]
- 9.Faizan M., Yu F., Rajput V.D., Minkina T., Hayat S. Brassinosteroids Signalling. Springer; Berlin/Heidelberg, Germany: 2022. Role of Brassinosteroids in Protein Folding Under High-Temperature Stress; pp. 259–268. [Google Scholar]
- 10.Baniwal S.K., Bharti K., Chan K.Y., Fauth M., Ganguli A., Kotak S., Mishra S.K., Nover L., Port M., Scharf K.-D. Heat stress response in plants: A complex game with chaperones and more than twenty heat stress transcription factors. J. Biosci. 2004;29:471–487. doi: 10.1007/BF02712120. [DOI] [PubMed] [Google Scholar]
- 11.Thakur M.P., van Der Putten W.H., Apon F., Angelini E., Vreš B., Geisen S. Resilience of rhizosphere microbial predators and their prey communities after an extreme heat event. Funct. Ecol. 2021;35:216–225. doi: 10.1111/1365-2435.13696. [DOI] [Google Scholar]
- 12.Szymańska R., Ślesak I., Orzechowska A., Kruk J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017;139:165–177. doi: 10.1016/j.envexpbot.2017.05.002. [DOI] [Google Scholar]
- 13.Hassan M.U., Chattha M.U., Khan I., Chattha M.B., Barbanti L., Aamer M., Iqbal M.M., Nawaz M., Mahmood A., Ali A. Heat stress in cultivated plants: Nature, impact, mechanisms, and mitigation strategies—A review. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2021;155:211–234. doi: 10.1080/11263504.2020.1727987. [DOI] [Google Scholar]
- 14.Lugtenberg B.J., Malfanova N., Kamilova F., Berg G. Plant growth promotion by microbes. Mol. Microb. Ecol. Rhizosphere. 2013;2:561–573. [Google Scholar]
- 15.Berg G., Alavi M., Schmidt C.S., Zachow C., Egamberdieva D., Kamilova F., Lugtenberg B. Biocontrol and osmoprotection for plants under salinated conditions. Mol. Microb. Ecol. Rhizosphere. 2013;1:561–573. [Google Scholar]
- 16.Naik K., Mishra S., Srichandan H., Singh P.K., Sarangi P.K. Plant growth promoting microbes: Potential link to sustainable agriculture and environment. Biocatal. Agric. Biotechnol. 2019;21:101326. doi: 10.1016/j.bcab.2019.101326. [DOI] [Google Scholar]
- 17.Ghosh S.K., Bera T., Chakrabarty A.M. Microbial siderophore–A boon to agricultural sciences. Biol. Control. 2020;144:104214. doi: 10.1016/j.biocontrol.2020.104214. [DOI] [Google Scholar]
- 18.Saha M., Sarkar S., Sarkar B., Sharma B.K., Bhattacharjee S., Tribedi P. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut. Res. 2016;23:3984–3999. doi: 10.1007/s11356-015-4294-0. [DOI] [PubMed] [Google Scholar]
- 19.Houghton J. Global warming. Rep. Prog. Phys. 2005;68:1343. doi: 10.1088/0034-4885/68/6/R02. [DOI] [Google Scholar]
- 20.Harvey L.D. Global Warming, The Hard Science. Routledge; Oxfordshire, UK: 2018. [Google Scholar]
- 21.Kerr R.A. Global warming is changing the world. Science. 2007;316:188–190. doi: 10.1126/science.316.5822.188. [DOI] [PubMed] [Google Scholar]
- 22.Kotak S., Larkindale J., Lee U., von Koskull-Döring P., Vierling E., Scharf K.-D. Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 2007;10:310–316. doi: 10.1016/j.pbi.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Drake F. Global Warming, The Science of Climate Change. Routledge; London, UK: 2014. [Google Scholar]
- 24.Memmott J., Craze P.G., Waser N.M., Price M.V. Global warming and the disruption of plant–pollinator interactions. Ecol. Lett. 2007;10:710–717. doi: 10.1111/j.1461-0248.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- 25.Trivedi P., Batista B.D., Bazany K.E., Singh B.K. Plant–microbiome interactions under a changing world: Responses, consequences and perspectives. New Phytol. 2022;234:1951–1959. doi: 10.1111/nph.18016. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka K.R., Van Houtan K.S. The recent normalization of historical marine heat extremes. PLoS Clim. 2022;1:e0000007. doi: 10.1371/journal.pclm.0000007. [DOI] [Google Scholar]
- 27.Meena M., Swapnil P., Zehra A., Aamir M., Dubey M., Goutam J., Upadhyay R. Plant-Microbe Interactions in Agro-Ecological Perspectives. Springer; Singapore: 2017. Beneficial Microbes for Disease Suppression and Plant Growth Promotion; pp. 395–432. [Google Scholar]
- 28.Zia R., Nawaz M.S., Siddique M.J., Hakim S., Imran A. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 2021;242:126626. doi: 10.1016/j.micres.2020.126626. [DOI] [PubMed] [Google Scholar]
- 29.Dai L., Zhang G., Yu Z., Ding H., Xu Y., Zhang Z. Effect of Drought Stress and Developmental Stages on Microbial Community Structure and Diversity in Peanut Rhizosphere Soil. Int. J. Mol. Sci. 2019;20:2265. doi: 10.3390/ijms20092265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tapera T., Akhtar S., Ahmad Z., Ejah w., Anjum S., Ahmad T., Mahboob W., Hafeez A., Labuschagne M., Rizwan Khan M. Physiological responses of wheat to drought stress and its mitigation approaches. Acta Physiol. Plant. 2018;40:80. [Google Scholar]
- 31.Curá J.A., Franz D.R., Filosofía J.E., Balestrasse K.B., Burgueño L.E. Inoculation with Azospirillum sp. and Herbaspirillum sp. Bacteria Increases the Tolerance of Maize to Drought Stress. Microorganisms. 2017;5:41. doi: 10.3390/microorganisms5030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill C.B., Li C. Genetic Improvement of Heat Stress Tolerance in Cereal Crops. Agronomy. 2022;12:1205. doi: 10.3390/agronomy12051205. [DOI] [Google Scholar]
- 33.Berg G. Plant–microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009;84:11–18. doi: 10.1007/s00253-009-2092-7. [DOI] [PubMed] [Google Scholar]
- 34.Bednarek P., Osbourn A. Plant-microbe interactions: Chemical diversity in plant defense. Science. 2009;324:746–748. doi: 10.1126/science.1171661. [DOI] [PubMed] [Google Scholar]
- 35.Thomas A.S.S., Pongprayoon W., Cheenkachorn K., Sriariyanun M. Plant-Microbe Interactions-Insights and Views for Applications in Sustainable Agriculture. Appl. Sci. Eng. Prog. 2022 doi: 10.14416/j.asep.2021.07.008. [DOI] [Google Scholar]
- 36.Postgate J.R. The Fundamentals of Nitrogen Fixation. Cambridge University Press; Cambridge, UK: 1982. [Google Scholar]
- 37.Peoples M.B., Herridge D.F., Ladha J.K. Management of Biological Nitrogen Fixation for the Development of More Productive and Sustainable Agricultural Systems. Springer; Berlin/Heidelberg, Germany: 1995. Biological nitrogen fixation: An efficient source of nitrogen for sustainable agricultural production? pp. 3–28. [Google Scholar]
- 38.Allahverdiyeva Y., Isojärvi J., Zhang P., Aro E.-M. Cyanobacterial oxygenic photosynthesis is protected by flavodiiron proteins. Life. 2015;5:716–743. doi: 10.3390/life5010716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giller K.E. Nitrogen Fixation in Tropical Cropping Systems. Cabi; Wallingford, UK: 2001. [Google Scholar]
- 40.Glick B.R., Cheng Z., Czarny J., Duan J. Promotion of plant growth by ACC deaminase-producing soil bacteria. New Perspect. Eur. J. Plant Pathol. 2007;119:329–339. doi: 10.1007/s10658-007-9162-4. [DOI] [Google Scholar]
- 41.Anli M., Baslam M., Tahiri A., Raklami A., Symanczik S., Boutasknit A., Ait-El-Mokhtar M., Ben-Laouane R., Toubali S., Rahou Y.A. Biofertilizers as strategies to improve photosynthetic apparatus, growth, and drought stress tolerance in the date palm. Front. Plant Sci. 2020;11:516818. doi: 10.3389/fpls.2020.516818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shekhawat K., Almeida-Trapp M., García-Ramírez G.X., Hirt H. Beat the heat: Plant-and microbe-mediated strategies for crop thermotolerance. Trends Plant Sci. 2022 doi: 10.1016/j.tplants.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 43.Shaffique S., Khan M.A., Imran M., Park Y., Wani S.H., Lee I.-J. Research progress in the field of microbial mitigation for drought stress in plants. Front. Plant Sci. 2022;13:870626. doi: 10.3389/fpls.2022.870626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mukhtar T., Ali F., Rafique M., Ali J., Afridi M.S., Smith D., Mehmood S., Souleimanov A., Jellani G., Sultan T. Biochemical Characterization and Potential of Bacillus safensis Strain SCAL1 to Mitigate Heat Stress in Solanum lycopersicum L. J. Plant Growth Regul. 2022:1–16. doi: 10.1007/s00344-021-10571-4. [DOI] [Google Scholar]
- 45.Jia H., Xi Z., Ma J., Li Y., Hao C., Lu M., Zhang Z.-Z., Deng W.-W. Endophytic bacteria from the leaves of two types of albino tea plants, indicating the plant growth promoting properties. Plant Growth Regul. 2022;96:331–343. doi: 10.1007/s10725-021-00779-5. [DOI] [Google Scholar]
- 46.Akhtar N., Wani A.K., Dhanjal D.S., Mukherjee S. Insights into the beneficial roles of dark septate endophytes in plants under challenging environment: Resilience to biotic and abiotic stresses. World J. Microbiol. Biotechnol. 2022;38:79. doi: 10.1007/s11274-022-03264-x. [DOI] [PubMed] [Google Scholar]
- 47.Park Y.-G., Mun B.-G., Kang S.-M., Hussain A., Shahzad R., Seo C.-W., Kim A.-Y., Lee S.-U., Oh K.Y., Lee D.Y. Bacillus aryabhattai SRB02 tolerates oxidative and nitrosative stress and promotes the growth of soybean by modulating the production of phytohormones. PLoS ONE. 2017;12:e0173203. doi: 10.1371/journal.pone.0173203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meena H., Ahmed M.A., Prakash P. Amelioration of heat stress in wheat, Triticum aestivum by PGPR (Pseudomonas aeruginosa strain 2CpS1) Biosci. Biotechnol. Res. 2015;8:171–174. [Google Scholar]
- 49.Bisht N., Mishra S.K., Chauhan P.S. Bacillus amyloliquefaciens inoculation alters physiology of rice (Oryza sativa L. var. IR-36) through modulating carbohydrate metabolism to mitigate stress induced by nutrient starvation. Int. J. Biol. Macromol. 2020;143:937–951. doi: 10.1016/j.ijbiomac.2019.09.154. [DOI] [PubMed] [Google Scholar]
- 50.Basu A., Prasad P., Das S.N., Kalam S., Sayyed R., Reddy M., El Enshasy H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability. 2021;13:1140. doi: 10.3390/su13031140. [DOI] [Google Scholar]
- 51.El-Sawah A.M., El-Keblawy A., Ali D.F.I., Ibrahim H.M., El-Sheikh M.A., Sharma A., Alhaj Hamoud Y., Shaghaleh H., Brestic M., Skalicky M. Arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria enhance soil key enzymes, plant growth, seed yield, and qualitative attributes of guar. Agriculture. 2021;11:194. doi: 10.3390/agriculture11030194. [DOI] [Google Scholar]
- 52.Issa A., Esmaeel Q., Sanchez L., Courteaux B., Guise J.-F., Gibon Y., Ballias P., Clément C., Jacquard C., Vaillant-Gaveau N. Impacts of Paraburkholderia phytofirmans strain PsJN on tomato (Lycopersicon esculentum L.) under high temperature. Front. Plant Sci. 2018;9:1397. doi: 10.3389/fpls.2018.01397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang S.-M., Khan A.L., Waqas M., Asaf S., Lee K.-E., Park Y.-G., Kim A.-Y., Khan M.A., You Y.-H., Lee I.-J. Integrated phytohormone production by the plant growth-promoting rhizobacterium Bacillus tequilensis SSB07 induced thermotolerance in soybean. J. Plant Interact. 2019;14:416–423. doi: 10.1080/17429145.2019.1640294. [DOI] [Google Scholar]
- 54.Rana S., Chauhan R., Walia A., Sharma G., Datt N. Beneficial microbes in agriculture under abiotic stress conditions: An overview. Pharma Innov. 2021;10:360–368. doi: 10.22271/tpi.2021.v10.i1e.5542. [DOI] [Google Scholar]
- 55.Ali S.Z., Sandhya V., Grover M., Linga V.R., Bandi V. Effect of inoculation with a thermotolerant plant growth promoting Pseudomonas putida strain AKMP7 on growth of wheat (Triticum spp.) under heat stress. J. Plant Interact. 2011;6:239–246. doi: 10.1080/17429145.2010.545147. [DOI] [Google Scholar]
- 56.Ali S.Z., Sandhya V., Grover M., Kishore N., Rao L.V., Venkateswarlu B. Pseudomonas sp. strain AKM-P6 enhances tolerance of sorghum seedlings to elevated temperatures. Biol. Fertil. Soils. 2009;46:45–55. doi: 10.1007/s00374-009-0404-9. [DOI] [Google Scholar]
- 57.Abd El-Daim I.A., Bejai S., Meijer J. Improved heat stress tolerance of wheat seedlings by bacterial seed treatment. Plant Soil. 2014;379:337–350. doi: 10.1007/s11104-014-2063-3. [DOI] [Google Scholar]
- 58.Maitra S., Pramanick B., Dey P., Bhadra P., Shankar T., Anand K. Soil Microbiomes for Sustainable Agriculture. Springer; Berlin/Heidelberg, Germany: 2021. Thermotolerant Soil Microbes and Their Role in Mitigation of Heat Stress in Plants; pp. 203–242. [Google Scholar]
- 59.Mitra D., Rodríguez A.M.D., Cota F.I.P., Khoshru B., Panneerselvam P., Moradi S., Sagarika M.S., Anđelković S., de los Santos-Villalobos S., Mohapatra P.K.D. Amelioration of thermal stress in crops by plant growth-promoting rhizobacteria. Physiol. Mol. Plant Pathol. 2021;115:101679. doi: 10.1016/j.pmpp.2021.101679. [DOI] [Google Scholar]
- 60.Evans J.R. Improving photosynthesis. Plant Physiol. 2013;162:1780–1793. doi: 10.1104/pp.113.219006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allakhverdiev S.I., Kreslavski V.D., Klimov V.V., Los D.A., Carpentier R., Mohanty P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008;98:541–550. doi: 10.1007/s11120-008-9331-0. [DOI] [PubMed] [Google Scholar]
- 62.Crafts-Brandner S.J., Salvucci M.E. Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol. 2002;129:1773–1780. doi: 10.1104/pp.002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Law R.D., Crafts-Brandner S.J. Inhibition and acclimation of photosynthesis to heat stress is closely correlated with activation of ribulose-1, 5-bisphosphate carboxylase/oxygenase. Plant Physiol. 1999;120:173–182. doi: 10.1104/pp.120.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qu Y., Sakoda K., Fukayama H., Kondo E., Suzuki Y., Makino A., Terashima I., Yamori W. Overexpression of both Rubisco and Rubisco activase rescues rice photosynthesis and biomass under heat stress. Plant Cell Environ. 2021;44:2308–2320. doi: 10.1111/pce.14051. [DOI] [PubMed] [Google Scholar]
- 65.Hasanuzzaman M., Nahar K., Alam M.M., Roychowdhury R., Fujita M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013;14:9643–9684. doi: 10.3390/ijms14059643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.As W., Galani S., Ashraf M., Foolad M. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007;61:199–223. doi: 10.1016/j.envexpbot.2007.05.011. [DOI] [Google Scholar]
- 67.Rivero R.M., Ruiz J.M., García P.C., López-Lefebre L.R., Sánchez E., Romero L. Resistance to cold and heat stress: Accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001;160:315–321. doi: 10.1016/S0168-9452(00)00395-2. [DOI] [PubMed] [Google Scholar]
- 68.Gulen H., Eris A. Effect of heat stress on peroxidase activity and total protein content in strawberry plants. Plant Sci. 2004;166:739–744. doi: 10.1016/j.plantsci.2003.11.014. [DOI] [Google Scholar]
- 69.Aliyari Rad S., Dehghanian Z., Asgari Lajayer B., Nobaharan K., Astatkie T. Mitochondrial Respiration and Energy Production Under Some Abiotic Stresses. J. Plant Growth Regul. 2021:1–15. doi: 10.1007/s00344-021-10512-1. [DOI] [Google Scholar]
- 70.Scafaro A.P., Fan Y., Posch B.C., Garcia A., Coast O., Atkin O.K. Responses of leaf respiration to heatwaves. Plant Cell Environ. 2021;44:2090–2101. doi: 10.1111/pce.14018. [DOI] [PubMed] [Google Scholar]
- 71.Jagadish S.K., Way D.A., Sharkey T.D. Plant heat stress: Concepts directing future research. Plant Cell Environ. 2021;44:1992–2005. doi: 10.1111/pce.14050. [DOI] [PubMed] [Google Scholar]
- 72.Essemine J., Ammar S., Bouzid S. Impact of heat stress on germination and growth in higher plants: Physiological, biochemical and molecular repercussions and mechanisms of defence. J. Biol. Sci. 2010;10:565–572. doi: 10.3923/jbs.2010.565.572. [DOI] [Google Scholar]
- 73.Schöffl F., Prandl R., Reindl A. Molecular responses to heat stress. Mol. Responses Cold Drought Heat Salt Stress High. Plants. 1999;83:93. [Google Scholar]
- 74.Zhu L., Li H., Thorpe M.R., Hocart C.H., Song X. Stomatal and mesophyll conductance are dominant limitations to photosynthesis in response to heat stress during severe drought in a temperate and a tropical tree species. Trees. 2021;35:1613–1626. doi: 10.1007/s00468-021-02140-9. [DOI] [Google Scholar]
- 75.Bharath P., Gahir S., Raghavendra A.S. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 2021;12:324. doi: 10.3389/fpls.2021.615114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iqbal N., Umar S., Khan N.A., Corpas F.J. Crosstalk between abscisic acid and nitric oxide under heat stress: Exploring new vantage points. Plant Cell Rep. 2021;40:1429–1450. doi: 10.1007/s00299-021-02695-4. [DOI] [PubMed] [Google Scholar]
- 77.Li X., Palta J.A., Liu F. Modulation of Stomatal Response by Elevated CO2 in Plants Under Drought and Heat Stress. Front. Plant Sci. 2022;13:843999. doi: 10.3389/fpls.2022.843999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumar M., Kour D., Yadav A.N., Saxena R., Rai P.K., Jyoti A., Tomar R.S. Biodiversity of methylotrophic microbial communities and their potential role in mitigation of abiotic stresses in plants. Biologia. 2019;74:287–308. doi: 10.2478/s11756-019-00190-6. [DOI] [Google Scholar]
- 79.Mylona P., Pawlowski K., Bisseling T. Symbiotic nitrogen fixation. Plant Cell. 1995;7:869. doi: 10.2307/3870043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Freitas V.F., Cerezini P., Hungria M., Nogueira M.A. Strategies to deal with drought-stress in biological nitrogen fixation in soybean. Appl. Soil Ecol. 2022;172:104352. doi: 10.1016/j.apsoil.2021.104352. [DOI] [Google Scholar]
- 81.Moynihan M.A., Goodkin N.F., Morgan K.M., Kho P.Y., Lopes dos Santos A., Lauro F.M., Baker D.M., Martin P. Coral-associated nitrogen fixation rates and diazotrophic diversity on a nutrient-replete equatorial reef. ISME J. 2022;16:233–246. doi: 10.1038/s41396-021-01054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goyal R.K., Schmidt M.A., Hynes M.F. Molecular biology in the improvement of biological nitrogen fixation by rhizobia and extending the scope to cereals. Microorganisms. 2021;9:125. doi: 10.3390/microorganisms9010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rädecker N., Pogoreutz C., Gegner H.M., Cárdenas A., Perna G., Geißler L., Roth F., Bougoure J., Guagliardo P., Struck U. Heat stress reduces the contribution of diazotrophs to coral holobiont nitrogen cycling. ISME J. 2022;16:1110–1118. doi: 10.1038/s41396-021-01158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Glick B.R. Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiol. Lett. 2005;251:1–7. doi: 10.1016/j.femsle.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Y.-F., He L.-Y., Chen Z.-J., Wang Q.-Y., Qian M., Sheng X.-F. Characterization of ACC deaminase-producing endophytic bacteria isolated from copper-tolerant plants and their potential in promoting the growth and copper accumulation of Brassica napus. Chemosphere. 2011;83:57–62. doi: 10.1016/j.chemosphere.2011.01.041. [DOI] [PubMed] [Google Scholar]
- 86.Wong W., Tan S., Ge L., Chen X., Yong J. Bacterial Metabolites in Sustainable Agroecosystem. Springer; Berlin/Heidelberg, Germany: 2015. The importance of phytohormones and microbes in biofertilizers; pp. 105–158. [Google Scholar]
- 87.Frankenberger W.T., Arshad M. Phytohormones in Soils: Microbial Production and Function. CRC Press; Boca Raton, FL, USA: 2020. [Google Scholar]
- 88.Hirsch A.M., Fang Y., Asad S., Kapulnik Y. The role of phytohormones in plant-microbe symbioses. Plant Soil. 1997;194:171–184. doi: 10.1023/A:1004292020902. [DOI] [Google Scholar]
- 89.Masood S., Zhao X.Q., Shen R.F. Bacillus pumilus promotes the growth and nitrogen uptake of tomato plants under nitrogen fertilization. Sci. Hortic. 2020;272:109581. doi: 10.1016/j.scienta.2020.109581. [DOI] [Google Scholar]
- 90.Zhang F., Dashti N., Hynes R., Smith D.L. Plant growth-promoting rhizobacteria and soybean [Glycine max (L.) Merr.] growth and physiology at suboptimal root zone temperatures. Ann. Bot. 1997;79:243–249. doi: 10.1006/anbo.1996.0332. [DOI] [Google Scholar]
- 91.Muneer S., Ahmad J., Bashir H., Qureshi M. Proteomics of nitrogen fixing nodules under various environmental stresses. Plant Omics. 2012;5:167–176. [Google Scholar]
- 92.Saha B.N., Roy S., Rakshit R. Soil Management For Sustainable Agriculture. Apple Academic Press; Waretown, NJ, USA: 2022. Managing Abiotic Stressed Agriculture through Microbes; pp. 123–141. [Google Scholar]
- 93.Belimov A.A., Dodd I.C., Safronova V.I., Dumova V.A., Shaposhnikov A.I., Ladatko A.G., Davies W.J. Abscisic acid metabolizing rhizobacteria decrease ABA concentrations in planta and alter plant growth. Plant Physiol. Biochem. 2014;74:84–91. doi: 10.1016/j.plaphy.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 94.Tiwari M., Kumar R., Min D., Krishna Jagadish S. Genetic and molecular mechanisms underlying root architecture and function under heat stress-A hidden story. Plant Cell Environ. 2022;45:771–788. doi: 10.1111/pce.14266. [DOI] [PubMed] [Google Scholar]
- 95.Garg G., Kumar S., Bhati S. Endophytes: Mineral Nutrient Management. Volume 3. Springer; Berlin/Heidelberg, Germany: 2021. Siderophore in Plant Nutritional Management: Role of Endophytic Bacteria; pp. 315–329. [Google Scholar]
- 96.Notununu I., Moleleki L., Roopnarain A., Adeleke R. Effects of plant growth-promoting rhizobacteria on the molecular responses of maize under drought and heat stresses: A review. Pedosphere. 2022;32:90–106. doi: 10.1016/S1002-0160(21)60051-6. [DOI] [Google Scholar]
- 97.Xu X., Wang Q., Li W., Hu T., Wang Q., Yin Y., Liu X., He S., Zhang M., Liang Y. Overexpression of SlBBX17 affects plant growth and enhances heat tolerance in tomato. Int. J. Biol. Macromol. 2022;206:799–811. doi: 10.1016/j.ijbiomac.2022.03.080. [DOI] [PubMed] [Google Scholar]
- 98.Prasad P., Staggenborg S., Ristic Z. Response of Crops to Limited Water: Understanding and Modeling Water Stress Effects on Plant Growth Processes. Volume 1. American Society of Agronomy, Inc.; Crop Science Society of America, Inc.; Soil Science Society of America, Inc.; Madison, WI, USA: 2008. Impacts of drought and/or heat stress on physiological, developmental, growth, and yield processes of crop plants; pp. 301–355. [Google Scholar]
- 99.Singh R.P., Ma Y., Shadan A. Perspective of ACC-deaminase producing bacteria in stress agriculture. J. Biotechnol. 2022;352:36–46. doi: 10.1016/j.jbiotec.2022.05.002. [DOI] [PubMed] [Google Scholar]
- 100.Gururani M.A., Upadhyaya C.P., Baskar V., Venkatesh J., Nookaraju A., Park S.W. Plant Growth-Promoting Rhizobacteria Enhance Abiotic Stress Tolerance in Solanum tuberosum Through Inducing Changes in the Expression of ROS-Scavenging Enzymes and Improved Photosynthetic Performance. J. Plant Growth Regul. 2013;32:245–258. doi: 10.1007/s00344-012-9292-6. [DOI] [Google Scholar]
- 101.Choi J., Roy Choudhury A., Park S.-Y., Oh M.-M., Sa T. Inoculation of ACC Deaminase-Producing Brevibacterium linens RS16 Enhances Tolerance against Combined UV-B Radiation and Heat Stresses in Rice (Oryza sativa L.) Sustainability. 2021;13:10013. doi: 10.3390/su131810013. [DOI] [Google Scholar]
- 102.Mukhtar T., Smith D., Sultan T., Seleiman M.F., Alsadon A.A., Ali S., Chaudhary H.J., Solieman T.H., Ibrahim A.A., Saad M.A. Mitigation of heat stress in Solanum lycopersicum L. by ACC-deaminase and exopolysaccharide producing Bacillus cereus: Effects on biochemical profiling. Sustainability. 2020;12:2159. doi: 10.3390/su12062159. [DOI] [Google Scholar]
- 103.Shekhawat K., Saad M.M., Sheikh A., Mariappan K., Al-Mahmoudi H., Abdulhakim F., Eida A.A., Jalal R., Masmoudi K., Hirt H. Root endophyte induced plant thermotolerance by constitutive chromatin modification at heat stress memory gene loci. EMBO Rep. 2021;22:e51049. doi: 10.15252/embr.202051049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Duc N.H., Csintalan Z., Posta K. Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants. Plant Physiol. Biochem. 2018;132:297–307. doi: 10.1016/j.plaphy.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 105.Khan M.A., Asaf S., Khan A.L., Jan R., Kang S.-M., Kim K.-M., Lee I.-J. Thermotolerance effect of plant growth-promoting Bacillus cereus SA1 on soybean during heat stress. BMC Microbiol. 2020;20:175. doi: 10.1186/s12866-020-01822-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cosoveanu A., Chowdhary K., Cabrera R., Sharma S. Endophytes. Springer; Singapore: 2021. Role of Phytohormones-Producing Fungal Endophytes in Plant–Microbial Interactions Under Stress; pp. 195–223. [Google Scholar]
- 107.Choudhary D.K., Kasotia A., Jain S., Vaishnav A., Kumari S., Sharma K.P., Varma A. Bacterial-Mediated Tolerance and Resistance to Plants Under Abiotic and Biotic Stresses. J. Plant Growth Regul. 2016;35:276–300. doi: 10.1007/s00344-015-9521-x. [DOI] [Google Scholar]
- 108.Binenbaum J., Weinstain R., Shani E. Gibberellin localization and transport in plants. Trends Plant Sci. 2018;23:410–421. doi: 10.1016/j.tplants.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 109.Atzorn R., Crozier A., Wheeler C., Sandberg G. Production of gibberellins and indole-3-acetic acid by Rhizobium phaseoli in relation to nodulation of Phaseolus vulgaris roots. Planta. 1988;175:532–538. doi: 10.1007/BF00393076. [DOI] [PubMed] [Google Scholar]
- 110.Siddique K., Belford R., Tennant D. Root: Shoot ratios of old and modern, tall and semi-dwarf wheats in a Mediterranean environment. Plant Soil. 1990;121:89–98. doi: 10.1007/BF00013101. [DOI] [Google Scholar]
- 111.Yamaguchi N., Ito T. JMJ histone demethylases balance H3K27me3 and H3K4me3 levels at the HSP21 locus during heat acclimation in Arabidopsis. Biomol. 2021;11:852. doi: 10.3390/biom11060852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qian Y., Chen C., Jiang L., Zhang J., Ren Q. Genome-wide identification, classification and expression analysis of the JmjC domain-containing histone demethylase gene family in maize. BMC Genom. 2019;20:256. doi: 10.1186/s12864-019-5633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oberkofler V., Bäurle I. Inducible epigenome editing probes for the role of histone H3K4 methylation in Arabidopsis heat stress memory. Plant Physiol. 2022;189:703–714. doi: 10.1093/plphys/kiac113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yamaguchi N., Matsubara S., Yoshimizu K., Seki M., Hamada K., Kamitani M., Kurita Y., Inagaki S., Suzuki T., Gan E.-S. Removal of repressive histone marks creates epigenetic memory of recurring heat in Arabidopsis. BioRxiv. 2020 doi: 10.1101/2020.05.10.086611. [DOI] [Google Scholar]
- 115.He K., Cao X., Deng X. Histone methylation in epigenetic regulation and temperature responses. Curr. Opin. Plant Biol. 2021;61:102001. doi: 10.1016/j.pbi.2021.102001. [DOI] [PubMed] [Google Scholar]
- 116.Zhongming Z., Wei L. How to Beat the Heat: Memory Mechanism Allows Plants to Adapt to Heat Stress. Nara Institute of Science and Technology; Nara, Japan: 2021. [Google Scholar]
- 117.Mittler R., Finka A., Goloubinoff P. How do plants feel the heat? Trends Biochem. Sci. 2012;37:118–125. doi: 10.1016/j.tibs.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 118.Wang Q.-L., Chen J.-H., He N.-Y., Guo F.-Q. Metabolic reprogramming in chloroplasts under heat stress in plants. Int. J. Mol. Sci. 2018;19:849. doi: 10.3390/ijms19030849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hu S., Ding Y., Zhu C. Sensitivity and responses of chloroplasts to heat stress in plants. Front. Plant Sci. 2020;11:375. doi: 10.3389/fpls.2020.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sharma M., Kumar P., Verma V., Sharma R., Bhargava B., Irfan M. Understanding plant stress memory response for abiotic stress resilience: Molecular insights and prospects. Plant Physiol. Biochem. 2022;179:10–24. doi: 10.1016/j.plaphy.2022.03.004. [DOI] [PubMed] [Google Scholar]
- 121.Cellini A., Spinelli F., Donati I., Ryu C.-M., Kloepper J.W. Bacterial volatile compound-based tools for crop management and quality. Trends Plant Sci. 2021;26:968–983. doi: 10.1016/j.tplants.2021.05.006. [DOI] [PubMed] [Google Scholar]
- 122.Gámez-Arcas S., Baroja-Fernández E., García-Gómez P., Muñoz F.J., Almagro G., Bahaji A., Sánchez-López Á.M., Pozueta-Romero J. Action mechanisms of small microbial volatile compounds in plants. J. Exp. Bot. 2022;73:498–510. doi: 10.1093/jxb/erab463. [DOI] [PubMed] [Google Scholar]
- 123.Mburu S., Koskey G., Njeru E., Maingi J. Revitalization of bacterial endophytes and rhizobacteria for nutrients bioavailability in degraded soils to promote crop production. AIMS Agric. Food. 2021;6:496–524. doi: 10.3934/agrfood.2021029. [DOI] [Google Scholar]
- 124.Lamaoui M., Jemo M., Datla R., Bekkaoui F. Heat and Drought Stresses in Crops and Approaches for Their Mitigation. Front. Chem. 2018;6:26. doi: 10.3389/fchem.2018.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Khan M.A., Asaf S., Khan A.L., Jan R., Kang S.-M., Kim K.-M., Lee I.-J. Extending thermotolerance to tomato seedlings by inoculation with SA1 isolate of Bacillus cereus and comparison with exogenous humic acid application. PLoS ONE. 2020;15:e0232228. doi: 10.1371/journal.pone.0232228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Onwe R.O., Onwosi C.O., Ezugworie F.N., Ekwealor C.C., Okonkwo C.C. Microbial trehalose boosts the ecological fitness of biocontrol agents, the viability of probiotics during long-term storage and plants tolerance to environmental-driven abiotic stress. Sci. Total Environ. 2022;806:150432. doi: 10.1016/j.scitotenv.2021.150432. [DOI] [PubMed] [Google Scholar]
- 127.Elnahal A.S., El-Saadony M.T., Saad A.M., Desoky E.-S.M., El-Tahan A.M., Rady M.M., AbuQamar S.F., El-Tarabily K.A. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: A review. Eur. J. Plant Pathol. 2022;162:759–792. doi: 10.1007/s10658-021-02393-7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the present study are available from the corresponding author upon request.