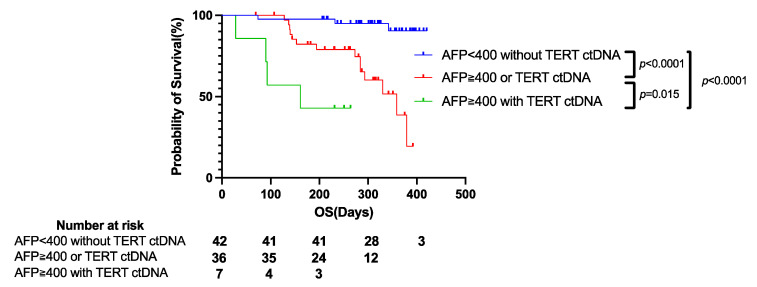
Figure 5.
TERT ctDNA mutation and AFP level stratify prognosis of u-HCC patients treated with combination immunotherapy. Total 85 u-HCC patients treated with Atezo/Bev were divided into three groups based on the AFP levels and the presence or absence of TERT ctDNA mutation (AFP levels ≥ 400 ng/mL with TERT ctDNA mutation, AFP levels ≥ 400 ng/mL or TERT ctDNA mutation, AFP levels < 400 ng/mL without TERT ctDNA mutation). The Kaplan–Meier curves of overall survival (OS) for each group. ctDNA, circulating tumor DNA; u-HCC, unresectable hepatocellular carcinoma; Atezo/Bev, Atezolizumab and bevacizumab.