Abstract
Simple Summary
One-third of the approximately 10 million deaths yearly caused by cancer worldwide are due to hepatobiliary, pancreatic, and gastrointestinal tumors. One primary reason for this high mortality is the lack of response of these cancers to pharmacological treatment. More than 100 genes have been identified as responsible for seven mechanisms of chemoresistance, but only a few of them play a critical role. These include ABC proteins (mainly MDR1, MRP1-6, and BCRP), whose expression pattern greatly determines the individual sensitivity of each tumor to pharmacotherapy.
Abstract
Hepatobiliary, pancreatic, and gastrointestinal cancers account for 36% of the ten million deaths caused by cancer worldwide every year. The two main reasons for this high mortality are their late diagnosis and their high refractoriness to pharmacological treatments, regardless of whether these are based on classical chemotherapeutic agents, targeted drugs, or newer immunomodulators. Mechanisms of chemoresistance (MOC) defining the multidrug resistance (MDR) phenotype of each tumor depend on the synergic function of proteins encoded by more than one hundred genes classified into seven groups (MOC1-7). Among them, the efflux of active agents from cancer cells across the plasma membrane caused by members of the superfamily of ATP-binding cassette (ABC) proteins (MOC-1b) plays a crucial role in determining tumor MDR. Although seven families of human ABC proteins are known, only a few pumps (mainly MDR1, MRP1-6, and BCRP) have been associated with reducing drug content and hence inducing chemoresistance in hepatobiliary, pancreatic, and gastrointestinal cancer cells. The present descriptive review, which compiles the updated information on the expression of these ABC proteins, will be helpful because there is still some confusion on the actual relevance of these pumps in response to pharmacological regimens currently used in treating these cancers. Moreover, we aim to define the MOC pattern on a tumor-by-tumor basis, even in a dynamic way, because it can vary during tumor progression and in response to chemotherapy. This information is indispensable for developing novel strategies for sensitization.
Keywords: ATP-binding cassette protein, anticancer drug, drug refractoriness, multidrug resistance, transport
1. Introduction
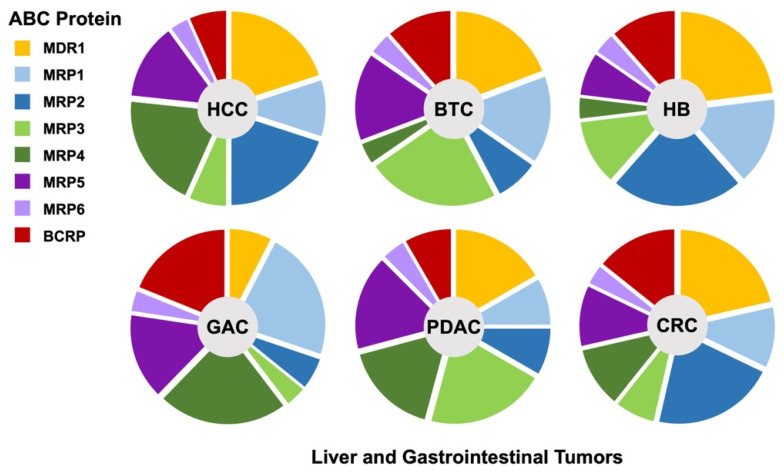
Hepatobiliary, pancreatic and gastrointestinal tumors account for approximately 36% of deaths due to cancer worldwide. One of the main reasons for this high mortality is their late diagnosis and high refractoriness to pharmacological treatments. In this way, most of these tumors are detected when curative approaches, such as surgical resection, are no longer advisable. The second cause of poor outcomes is their high refractoriness to pharmacological treatments, i.e., classical chemotherapy, targeted drugs, and newer immunomodulators, which may constitute the only therapeutical option for these patients with advanced cancers. More than 100 genes involved in the lack of response to drug therapy have been identified and classified into seven groups of mechanisms of chemoresistance (MOC) [1,2,3]. Among them, the efflux of active agents from cancer cells across the plasma membrane through members of the superfamily of ATP-binding cassette (ABC) proteins plays a crucial role in drug resistance mechanisms categorized as MOC-1b. Although there are seven families of ABC proteins in humans (from ABCA to ABCG), only members of the ABCB, ABCC, and ABCG families have been clearly associated with anticancer drug export (Table 1) [4]. Thus, the first member related to chemoresistance was named multidrug resistance protein 1 (MDR1), also known as P-glycoprotein or P-gp (gene symbol ABCB1). This pump can transport a large variety of anticancer drugs and is highly expressed in many tumors of the digestive system (Table 1). Several members of the ABCC family, known as multidrug resistance-associated proteins (MRPs), have also been associated with the lack of response to these tumors, namely MRP1 (ABCC1), MRP2 (ABCC2), MRP3 (ABCC3), MRP4 (ABCC4), MRP5 (ABCC5), and MRP6 (ABCC6). Although there is some overlap regarding substrate-specificity, there are also significant differences in this respect (Table 1). Moreover, their expression in these tumors shows characteristic patterns (Figure 1). Finally, there is a member of the ABCG family, the breast cancer resistance protein or BCRP (ABCG2), which plays a relevant role in the transport of anticancer drugs and hence in the chemoresistance of digestive cancers (Table 1). MDR1 and MRP1-6 are full transporters, whereas ABCG2 is a half-transporter that requires dimerization to become functionally active. In addition to their basal expression in naïve tumors, a common feature in resistance development is their upregulation in response to treatment [4]. Nevertheless, there is also the possibility of manipulating their expression [5] and function [6]. The present descriptive review compiles updated information on the expression of ABC proteins involved in the multidrug resistance (MDR) phenotype of liver and gastrointestinal cancers.
Table 1.
ABC proteins involved in the transport of common antitumor drugs used in the treatment of hepatobiliary, pancreatic, and gastrointestinal tumors.
| Pharmacological Groups | Drugs | Cancers | ABC Pumps | ||
|---|---|---|---|---|---|
| Alkylating Drugs | Cyclophosphamide | HB | MRP1, 4 | ||
| Anthracyclines | Doxorubicin | GAC, HB, HCC | MDR1 | MRP1, 2, 6 | BCRP |
| Camptothecins | Irinotecan | BTC, CRC, GAC, HB, PDAC | MDR1 | MRP1, 2, 4, 6 | BCRP |
| TKIs | Sorafenib | HCC | MDR1 | MRP1, 2, 3 | BCRP |
| Regorafenib | CRC, HCC | MDR1 | MRP2 | ||
| Lenvatinib | HCC | MDR1 | MRP2 | ||
| Cabozantinib | HCC | MDR1 | MRP2 | ||
| Platinum Derivatives | Cisplatin | BTC, GAC, HB, HCC | MRP2, 6 | ||
| Oxaliplatin | BTC, CRC, GAC, PDAC | MRP2, 6 | |||
| Podophyllotoxins | Etoposide | BTC, HB | MDR1 | MRP1, 2 | |
| Pyrimidines | 5-FU | BTC, CRC, GAC, HB, HCC, PDAC | MRP1, 4, 5 | BCRP | |
| Gemcitabine | BTC, PDAC | MDR1 | MRP2, 4, 5, 6 | ||
| Taxanes | Docetaxel | GAC | MDR1 | MRP1 | |
| Vinca Alkaloids | Vinblastine | HB | MDR1 | MRP1, 2 | |
5-FU, 5-Fluorouracil; BCRP, breast cancer resistance protein; BTC, biliary tract cancer; CRC, colorectal carcinoma; GAC, gastric adenocarcinoma; HB, hepatoblastoma; HCC, hepatocellular carcinoma; MDR, multidrug resistance protein; MRP, multidrug resistant-associated protein; PDCA, pancreatic ductal adenocarcinoma; TKI, tyrosine kinase inhibitor.
Figure 1.
Expression pattern of ABC proteins involved in chemoresistance of liver and gastrointestinal cancers. HCC, hepatocellular carcinoma; BTC, biliary tract cancer; HB, hepatoblastoma; GAC, gastric adenocarcinoma; PDAC, pancreatic ductal adenocarcinoma; CRC, colorectal carcinoma.
2. Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC), which derives from hepatocytes, is the most frequent primary liver cancer, causing more than 800,000 deaths per year worldwide, i.e., more than 8% of deaths due to cancer (Global Cancer Observatory; http://gco.iarc.fr accessed on 1 June 2022). Systemic drug therapy is the only therapeutic option for most patients with unresectable advanced HCC. However, one important feature accounting for the high mortality of HCC is its marked drug refractoriness mainly to classical chemotherapy, whose current use is limited to locoregional therapies, such as transarterial chemoembolization (TACE) with doxorubicin, cisplatin, or 5-fluorouracil (5-FU) in a small group of patients. Moreover, alternative treatments based on tyrosine kinase inhibitors (TKIs), such as sorafenib, lenvatinib, regorafenib, and cabozantinib, or even immunotherapy, provide very modest beneficial effects [7]. This is in part due to drug efflux from cancer cells through ABC proteins, which play a crucial role in the sensitivity of pharmacotherapy in HCC (Table 2).
Table 2.
Role of ABC proteins in the chemoresistance of hepatocellular carcinoma (HCC).
| Change | Drugs Affected | Impact | Ref. |
|---|---|---|---|
| MDR1 upregulation | Sorafenib | Reduced OS | [8,9] |
| Doxorubicin | Worse prognosis | [10] | |
| MDR1 variant rs1045642 | Sorafenib | Better clinical evolution | [11] |
| ABCB5 upregulation | Doxorubicin | Decreased cell sensitivity in vitro | [12] |
| MRP1 upregulation | Sorafenib | Worse response | [13] |
| MRP2 upregulation | Sorafenib | Decreased cell sensitivity in vitro | [14] |
| MRP3 upregulation | Sorafenib | Decreased cell sensitivity in vitro | [15] |
| MRP4 upregulation | Cisplatin | Decreased cell sensitivity in vitro | [16] |
| MRP5 upregulation | 5-FU, cisplatin, doxorubicin | Decreased cell sensitivity in vitro | [17,18,19] |
| BCRP upregulation | Doxorubicin | Decreased cell sensitivity in vitro | [20] |
| Sorafenib | Worse response | [9,21] | |
| BCRP variants rs2231137 and rs2231142 | Sorafenib | Better clinical evolution | [11] |
| ABCF1 upregulation | Cisplatin, doxorubicin | Decreased cell sensitivity in vitro and in vivo | [22] |
5-FU, 5-Fluorouracil; BCRP, breast cancer resistance protein; MDR, multidrug resistance protein; MRP, multidrug resistant-associated protein; OS, overall survival.
2.1. MDR1 in Hepatocellular Carcinoma
MDR1, or P-glycoprotein, is expressed in the canalicular membrane of hepatocytes [23], where it exports amphiphilic cations, endogenous steroids, and xenobiotics into bile. MDR1 expression is highly variable among HCC tumors. Marked upregulation of MDR1 has been reported in some cases [24], whereas, in many others, MDR1 abundance (both mRNA and protein) is lower in the tumor than in adjacent liver tissue [25]. HCC-derived cell lines also show marked variability in MDR1 expression. Most of these cell lines express MDR1 only at low levels, whereas in a few of them, such as HuH-7 and Hep3B, this pump is highly expressed [26]. In the clinical setting, MDR1 expression is associated with short overall survival (OS) of HCC patients [8]. There is an inverse relationship between MDR1 expression levels in the tumor and the response to systemic chemotherapy of advanced HCC [25]. This is consistent with the fact that MDR1 is considered an important factor in determining the response of HCC to sorafenib [9]. Other TKIs approved by the FDA for the treatment of HCC, such as regorafenib and lenvatinib, are also MDR1 substrates [27,28]. Elevated MDR1 expression in the tumor has been associated with lower accumulation of doxorubicin and worse prognosis in patients treated with this drug, making doxorubicin scarcely effective against HCC [10].
2.2. MRP1 in Hepatocellular Carcinoma
Under basal conditions, healthy hepatocytes express MRP1 at very low, almost undetectable levels in the basolateral membrane [29]. However, in HCC, variable MRP1 expression has been reported. Some authors have found overexpression of this transporter in tumors compared to the surrounding tissue [30], while no difference [31] or even undetectable MRP1 protein levels [32] have been reported by others. Despite the well-known ability of MRP1 to export many antitumor drugs [33], there is scarce information on the role of this pump in the overall refractoriness to TKIs used in the treatment of HCC. In this sense, sorafenib seems to induce MRP1 expression in HCC-derived cells, presumably through ubiquitin peptidase 22 (UPS22) upregulation [13]. Consistently, positive staining of UPS22 and MRP1, assessed by immunohistochemistry, correlated with each other in sorafenib-resistant tumors, but not in sorafenib-sensitive ones [13].
2.3. MRP2 in Hepatocellular Carcinoma
MRP2 is highly expressed in the canalicular membrane of hepatocytes, where it plays an essential role in liver detoxification and chemoprotection processes [34]. MRP2 is also highly expressed in HCC [35] and, therefore, could contribute to its MDR phenotype. Indeed, MRP2 modulates the intracellular accumulation of sorafenib [14] due to the extrusion of the drug itself or its metabolite sorafenib glucuronide [36]. Moreover, MRP2 can also export regorafenib [37], cabozantinib [38], and probably lenvatinib [39].
2.4. MRP3 in Hepatocellular Carcinoma
Although with high individual variability, MRP3 expression shows a trend of decreasing levels in HCC compared with adjacent liver tissue [27,33,34]. Thus, in a recent study, only 18.8% of HCC (15 out of 80) showed positive staining for MRP3 by immunohistochemistry [40], suggesting a minor role of this ABC transporter in HCC chemoresistance. Nevertheless, a relationship between MRP3-mediated efflux and sensitivity to sorafenib in HCC cells has been reported [15].
2.5. MRP4 in Hepatocellular Carcinoma
In healthy hepatocytes, MRP4 is poorly expressed in their basolateral membrane [17]. In contrast, this pump is upregulated in cholestatic liver diseases, favoring the regurgitation of cholephilic compounds toward the sinusoidal blood. Similarly, MRP4 is also upregulated in HCC [41]. MRP4 expression is also high in cell lines of hepatic origins, such as HuH-7 [42] and HepaRG [43]. MRP4 mediates the extrusion of xenobiotics together with reduced glutathione (GSH) [17]. Studies performed on PLC/PRF5 cells exposed to sorafenib indicated that MRP4 is not involved in HCC refractoriness to this TKI [15]. Although cisplatin is not a known substrate of MRP4, in vitro studies have shown that cisplatin-resistant HCC cells exhibit MRP4 upregulation [16], which can be involved in developing cross-resistance.
2.6. MRP5 in Hepatocellular Carcinoma
MRP5 is expressed in the basal plasma membrane of many epithelial cells, including hepatocytes [44]. Some authors have described an increased MRP5 expression in untreated HCC compared to adjacent non-tumor liver tissue [18, 41]. In contrast, other studies have failed to detect MRP5 in either tumor or healthy liver parenchyma [45]. MRP5 is unambiguously detected (both mRNA and protein) in HCC-derived cell lines such as HuH-7, Hep3B, and HLF [39,40,44]. Some reports have also shown that in cisplatin- and doxorubicin-resistant HCC cells, MRP5 expression is higher than in wild-type cells [16,19]. Typical endogenous substrates of MRP5 are cyclic nucleotides, such as cAMP and cGMP [46]. In addition, this transporter can mediate the export of 5-FU, thus reducing the sensitivity of HCC cells to this drug [47]. However, so far, no clinical data on the relevance of MRP5 in the lack of response of HCC to chemotherapy or TKIs are available.
2.7. BCRP in Hepatocellular Carcinoma
BCRP is expressed in the apical membrane of hepatocytes [48] and in a very heterogeneous way in HCC-derived cells. Although some studies have reported non-significant upregulation of ABCG2 mRNA in HCC [41], in general, BCRP expression is higher in tumor tissue than in adjacent non-tumor liver tissue and healthy hepatocytes [49,50]. Moreover, ABCG2 mRNA levels in HCC-derived cells are higher in undifferentiated than in differentiated cell lines [49]. ABCG2 expression is typically very high in the so-called side-population (SP) of stem cells, whose chemoresistance is stronger than those without stemness characteristics [51]. Thus, BCRP is a potential marker for liver cancer stem cells (LCSC) [52,53]. Interestingly, BCRP expression is closely related to the initiation, proliferation, metastasis, and chemoresistance of HCC. Increased BCRP expression has been correlated with reduced OS in HCC of elderly patients [50]. Surprisingly, other studies have reported that HCC patients with low BCRP expression have a significantly shorter OS and recurrence-free survival time after treatment with epirubicin or cisplatin, alone or in combination with 5-FU [54]. ABCG2 expression, which can be modulated by AKT signaling, can significantly influence the efflux from HCC cells of drugs such as doxorubicin, therefore hindering their efficacy [54]. In addition, the development in HCC cells of an LCSC phenotype, in which ABCG2 upregulation is prominent, is associated with malignant traits, such as increased proliferation, migration, and invasion [53]. It is noteworthy that these features can be diminished by ABCG2 downregulation [52]. BCRP is one of the most relevant ABC proteins in determining the response of HCC patients to sorafenib [9]. BCRP plays a dominant role in sorafenib efflux [55], and it has been proposed as a predictor of HCC response to this drug [21]. In addition, HCC patients who carry the genetic variants rs2231137 (c.34G>A, p.Val12Met) and rs2231142 (c.421C>G/A, p.Gln141Glu/Lys) of ABCG2 and rs1045642 (c.3645T>G, p.Ile1215Met) of ABCB1 have lower plasma levels of sorafenib and show better progression-free survival [11]. However, the presence of these variants does not affect the pharmacokinetics of lenvatinib.
2.8. Other ABC Pumps in Hepatocellular Carcinoma
The involvement of other ABC proteins, such as ABCA2, ABCA3, ABCA6, ABCA8, MRP6, and MRP7 (ABCC10), in the development of the MDR phenotype in HCC, is considered scarcely relevant [56]. ABCB5 and ABCF1 are LCSC markers. Cell subpopulations with stemness properties, obtained from biopsies of HCC patients, express higher levels of ABCB5 than the surrounding non-tumor tissue [57]. Using Hep3B cells, the overexpression of ABCB5 has been related to the development of doxorubicin resistance [12]. ABCF1 is a hepatic oncofetal protein upregulated in HCC, which has recently been reported to promote resistance to cisplatin and doxorubicin using both in vitro and in vivo models [22].
3. Biliary Tract Cancer
Biliary tract cancer (BTC) refers to a group of rare and aggressive malignancies arising in the biliary tree that comprises cholangiocarcinoma (CCA), gallbladder cancer (GBC), and ampullary cancer. Options of systemic chemotherapy for treating patients with advanced BTC are similar in all cases, including in the first-line combinations of gemcitabine and cisplatin or oxaliplatin plus 5-FU (FOLFOX) or capecitabine (CAPOX). In the second-line setting, alternatives, such as FOLFIRINOX (5-FU, irinotecan, and oxaliplatin) and etoposide toniribate, have been assayed. Finally, novel targeted drugs and immunotherapy offer hope for the future of these patients, although no efficient therapy currently exists [58]. As part of the BTC “transportome”, defined as the set of transporters expressed at a given moment in the tumor, which is an essential element for defining its MDR phenotype, ABC proteins play a crucial role [59] (Table 3).
Table 3.
Role of ABC proteins in chemoresistance of biliary tract cancer (BTC).
| Change | Drugs Affected | Impact | Ref. |
|---|---|---|---|
| MDR1 upregulation | Gemcitabine | Reduced response | [60] |
| MRP1 upregulation | 5-FU, Gemcitabine | Decreased cell sensitivity in vitro | [61,62] |
| Cisplatin, Gemcitabine | Worse prognosis | [63,64] | |
| MRP3 downregulation | 5-FU | Increased cell sensitivity in vitro | [65] |
| MRP5 downregulation | Gemcitabine | Increased cell sensitivity in vitro | [66] |
| MRP6 downregulation | Gemcitabine | Increased cell sensitivity in vitro | [66] |
| MDR1, MRP3, and BCRP upregulation | 5-FU | Reduced response | [67] |
5-FU, 5-Fluorouracil; BCRP, breast cancer resistance protein; MDR, multidrug resistance protein; MRP, multidrug resistant-associated protein.
3.1. MDR1 in Biliary Tract Cancer
The presence of MDR1 in the apical plasma membrane of the bile duct and gallbladder epithelial cells suggests that this export pump plays a physiological role in preventing the accumulation of endogenous and xenobiotic compounds secreted into bile in cells of the biliary tree by exporting them back to bile [23,68]. MDR1 expression levels are maintained in CCA and GBC [41,68] but are usually decreased in poorly differentiated tumors [69]. Accordingly, MDR1 is considered a contributing factor to the MDR phenotype of these tumors [70]. In fact, in patients with GBC, high MDR1 levels have been associated with increased resistance to gemcitabine [60].
3.2. MRP1 in Biliary Tract Cancer
Although MRP1 is not detected in normal cholangiocytes and gallbladder epithelium [71], its expression has been found elevated in some cases of intrahepatic CCA (iCCA) [63]. MRP1 has been shown to confer resistance to gemcitabine in CCA-derived cells [61]. Several studies have also associated MRP1 downregulation with increased sensitivity to 5-FU of CCA-derived cells in vitro [62,72]. Moreover, in patients with iCCA or GBC who received adjuvant chemotherapy, their poor prognosis was associated with high MRP1 mRNA levels in these tumors [63,64].
3.3. MRP2 in Biliary Tract Cancer
MRP2 is expressed in the apical membrane of cholangiocytes and gallbladder epithelial cells, where it plays a role in the barrier for anionic metabolites present in bile [71]. In a study carried out in 2008, it was suggested that MRP2 is not relevant in BTC chemoresistance because this protein was detected by immunohistochemistry in only 4 out of 14 GBC analyzed and was not detectable in any CCA [73]. However, subsequent studies, including a more significant number of cases, suggested the opposite conclusion because mRNA expression was found elevated in 28 out of 55 iCCA [63], and reactivity by immunohistochemistry was observed in most (96.3%) of the 56 CCA analyzed. A higher expression was seen in well or moderately differentiated tumors [40]. Moreover, MRP2 expression was found in half (53.1%) of the 143 GBC samples analyzed in another study [74]. Although marked MRP2 expression has not been found in human GBC cell lines [66,75], this is enhanced by incubation with cisplatin [76]. Moreover, MRP2 contributes to resistance to cisplatin, oxaliplatin, and gemcitabine in other types of tumors [77,78,79], which justifies the need to look further into its role in BTC resistance.
3.4. MRP3 in Biliary Tract Cancer
Under physiological conditions, MRP3 is expressed in the basolateral membrane of cholangiocytes [80] and gallbladder epithelial cells [71]. MRP3 is also markedly expressed in BTC. In a study carried out in 2008 [73], positive staining by immunohistochemistry was found in 13 of 14 GBC and 4 of 7 CCA. More recently, other studies have reported intense MRP3 expression in 44.5% of 56 CCAs [40] and in 74.5% of 59 GBCs, which was higher in more differentiated tumors [81]. MRP3 expression is also elevated in human CCA-derived cells [75]. Moreover, it has been demonstrated that MRP3 plays an important role in CCA chemoresistance, and, accordingly, it has been proposed as a target for chemosensitization [65].
3.5. MRP4, MRP5, and MRP6 in Biliary Tract Cancer
Although MRP4 can transport nitrogenous bases and nucleoside derivatives used against BTC, such as 5-FU and gemcitabine, its low expression in healthy biliary epithelium [82] and, although somewhat higher, in CCA [41] suggests that this pump has a minor role in BTC chemoresistance.
Tissue mRNA analyses revealed that MRP5 is ubiquitously expressed. High levels of this export pump able to transport derivatives of nitrogenous base and nucleosides have been found in CCA [41] and CCA-derived cell lines [66]. The exposure of HuCCT1 and KMBC cells, from iCCA and extrahepatic CCA (eCCA), respectively, to gemcitabine enhanced MRP5 expression. In contrast, MRP5 knockdown significantly increased gemcitabine cytotoxicity in KMBC cells [66]. However, an association between MRP5 expression and drug response in BTC patients has not been described yet.
The liver is one of the tissues with the highest expression levels of MRP6. Nevertheless, it is not clear whether this pump is expressed in cholangiocytes. Moreover, MRP6 has not been detected in gallbladder epithelium [83]. In contrast, HuCCT1 cells, derived from iCCA, show high mRNA and protein MRP6 levels, while no expression was detected in KMBC cells derived from eCCA. Gemcitabine upregulates MRP6 in HuCCT1 cells, and its knockdown enhances the sensitivity to this drug [66].
3.6. BCRP in Biliary Tract Cancer
BCRP is expressed in the apical membrane of cholangiocytes [84] and gallbladder epithelial cells [85], suggesting a protective role against potentially toxic compounds present in bile. Immunohistochemical analysis of 41 GBC specimens showed apical staining in well-differentiated tumors, whereas in poorly differentiated tumors, part of the signal was localized intracellularly [85]. Increased resistance to 5-FU has been described in CCA patients with high expression of BCRP, MDR1, and MRP3 [67].
4. Hepatoblastoma
Hepatoblastoma (HB) is the most common liver cancer in children and is treated with surgery and adjuvant or neoadjuvant antitumor chemotherapy [86]. Standard first-line chemotherapy is based on cisplatin plus doxorubicin. Although the response is better than that of HCC, it is not infallible because about 20% of HB patients do not respond to treatment and have a poor prognosis [87]. Other platinum and anthracycline derivatives, as well as other drugs such as etoposide, TKIs, Vinca alkaloids, 5-FU, irinotecan, and nitrogen mustards, are used as second-line treatment for refractory HB [88]. The bioavailability of some of these drugs is dependent on ABC pumps (Table 4).
Table 4.
Role of ABC proteins in chemoresistance of hepatoblastoma (HB).
| Protein Change | Drugs Affected | Impact | Ref. |
|---|---|---|---|
| MDR1 upregulation | Doxorubicin | Decreased cell sensitivity in vitro | [89] |
| MRP1 upregulation | Doxorubicin | Reduced response | [33] |
| MRP2 upregulation | Cisplatin | Reduced response | [90] |
MDR, multidrug resistance protein; MRP, multidrug resistant-associated protein.
4.1. MDR1 in Hepatoblastoma
MDR1 expression is usually high in HB [91] and can be further enhanced during drug treatment [91,92]. HB-derived cell lines exhibit a wide range of MDR1 expression [26], which is upregulated by incubating these cells with cisplatin or doxorubicin [92,93]. Similarly, mouse HB xenografts showed increased levels of MDR1 expression following treatment with doxorubicin [94] and cisplatin [95]. MDR1 confers resistance to anthracyclines in HB, as has been demonstrated in HepG2 cells overexpressing this pump [89]. Other drugs used in the second-line treatment of HB, such as etoposide, irinotecan, Vinca alkaloids, and sorafenib are also MDR1 substrates. Accordingly, upregulation of this ABC protein in HB may reduce its response to these drugs [88].
4.2. MRP1, MRP2, and MRP3 in Hepatoblastoma
MRP1 is highly expressed in HB compared to the surrounding liver tissue [88], as well as in HB-derived cell lines, such as HepG2 [41,96], HuH-6, and HepT1 [97]. However, there are discrepant results on the changes in MRP1 expression induced by the pharmacological treatment [98]. MRP1 plays an important role in anthracycline resistance [33] and is upregulated in HuH-6 cell spheroids, which decreases their sensitivity to doxorubicin [97]. Furthermore, the sensitivity to this drug was enhanced in HepG2 cells after silencing MRP1 [99].
MRP2 expression is high in HB [41] and is not changed in response to the treatment of these patients with cisplatin and doxorubicin [98]. HB-derived cell lines also express MRP2 [97]. Moreover, cisplatin-resistant HepG2 cells exhibited increased MRP2 levels [100]. Of note, MRP2 expression has been associated with the lack of response to cisplatin both in vitro [26] and in patients [90]. MRP2 could be involved in the resistance of HB to substrates of this pump, such as anthracyclines, irinotecan, sorafenib, etoposide, and Vinca alkaloids.
The levels of MRP3 expression are similar in healthy liver and HB tumor samples from untreated or standard chemotherapy-treated patients [98]. However, MRP3 has been found upregulated in HepG2 cells after exposure to cisplatin [41,101]. In experiments using cell models of MRP3 expression, this pump was able to transport sorafenib [15] and etoposide [102].
4.3. MRP4 and MRP5 in Hepatoblastoma
Although MRP4 could be involved in HB resistance to irinotecan, cyclophosphamide, 5-FU, and doxorubicin [88], its relevance is probably minor because MRP4 expression in healthy liver and HB is similarly low [41]. In contrast, HepG2 cells have high levels of MRP4 expression [103], which is further increased after the incubation with cisplatin [41].
MRP5 can determine tumor sensitivity to 5-FU [104] or anthracyclines [105]. However, its relevance in HB is uncertain. There are some discrepant results regarding MRP5 expression in HB. Previous determinations in a small number of patients reported no significant differences between HB compared to healthy liver tissue [41]. However, more recent reports suggested the existence of upregulated MRP5 expression in HB [88]. Moreover, the exposure of HepG2 cells to cisplatin did not further enhance their already elevated MRP5 expression [88].
4.4. BCRP in Hepatoblastoma
Although some studies have reported higher BCRP expression levels in HB than in healthy liver tissue, both at the mRNA and protein levels [41,98], the story is unclear because BCRP expression in the plasma membrane of HB cells has been reported to be low [98]. Another study has also found a decreased abundance of ABCG2 mRNA in HB [88]. Moreover, BCRP expression was increased in the tumors after treating HB patients with cisplatin plus doxorubicin [98]. These findings were consistent with those obtained in HB-derived HuH-6 cells after short-term exposure to both drugs individually [88]. As anthracyclines, irinotecan, etoposide, sorafenib, and 5-FU are BCRP substrates, the expression of this ABC protein could affect the response of HB patients to these drugs [88].
4.5. Other ABC Pumps in Hepatoblastoma
The role of other ABC proteins in HB resistance is poorly understood. MRP6 and MRP7 are expressed in HB-derived cells and upregulated after cisplatin treatment [41,106]. These pumps can participate in resistance to etoposide, cisplatin, irinotecan, and anthracyclines [107,108].
5. Gastric Adenocarcinoma
Gastric adenocarcinoma (GAC) is a lethal type of cancer, accounting for approximately 770,000 deaths per year worldwide, which constitutes 7.7% of deaths due to cancer. The lack of availability of efficient pharmacological treatment for advanced GAC, either as neoadjuvant or adjuvant chemotherapy to surgical resection or radiotherapy [1,109] justifies the high mortality of this disease. As first-line treatment, the most used chemotherapeutic regimens include 5-FU, leucovorin, oxaliplatin, and perioperative docetaxel (FLOT) [110], and the combination of epirubicin, cisplatin, and 5-FU (ECF) [111]. However, other newer strategies such as immunotherapy or anti-VEGF therapy are also available [112]. Second-line treatments include classical drugs, such as docetaxel, irinotecan, as well as newer antibodies against tyrosine kinase receptors (TKRs) (trastuzumab and cetuximab), and some TKIs (erlotinib and gefitinib) [113]. The activity of several ABC proteins reduces the sensitivity to many of these drugs (Table 5).
Table 5.
Role of ABC proteins in chemoresistance of gastric adenocarcinoma (GAC).
| Change | Drugs Affected | Impact | Ref. |
|---|---|---|---|
| MDR1 upregulation | Cisplatin, epirubicin | Worse response | [114,115] |
| Cisplatin, oxaliplatin, epirubicin | Decreased cell sensitivity in vitro | [116,117,118] | |
| MRP1 upregulation | Cisplatin | Worse response | [119] |
| MRP4 downregulation | 5-FU | Increased cell sensitivity in vitro | [120] |
| Cisplatin | Increased cell sensitivity in vitro | [121] | |
| MRP4 upregulation | Dasatinib | Decreased cell sensitivity in vitro and in vivo | [122] |
| BCRP upregulation | Cisplatin, 5-FU | Reduced OS | [123,124] |
5-FU, 5-Fluorouracil; BCRP, breast cancer resistance protein; MDR, multidrug resistance protein; MRP, multidrug resistant-associated protein; OS, overall survival.
5.1. MDR1 in Gastric Adenocarcinoma
The relevance of MDR1 in GAC chemoresistance is unclear. MDR1 is barely detectable in normal gastric mucosa, either by mRNA expression analysis [125] or immunohistochemistry [126]. Some studies have reported enhanced MDR1 expression in the early stages of GAC development, associating such expression with a poor prognosis due to increased resistance to chemotherapy [127,128]. However, other authors have found intracellular localization of the protein in GAC-derived cells, which precludes any role of the pump as a drug exporter [129]. Elevated MDR1 expression has been observed in GACs from patients classified as poor responders to platinum-based therapy alone or in combination with epirubicin [114,115]. This was surprising because the cytotoxic activities of these drugs were not expected to be affected by MDR1 expression levels [130]. Nevertheless, studies carried out using in vitro cellular models have demonstrated the involvement of MDR1 in GAC resistance to chemotherapeutic treatment with oxaliplatin [116], cisplatin [117], and epirubicin [118]. This points to MDR1 as a potential target for gene therapy to improve the response to the pharmacological treatment of patients with advanced GAC.
5.2. MRP1 in Gastric Adenocarcinoma
MRP1 is highly expressed in gastric mucosa and GAC [131,132]. The expression levels of this protein have been extensively investigated in several studies, which have reported elevated MRP1 expression in a variable proportion of cases ranging from 12 to 89% of GACs analyzed [132,133,134]. In most GAC specimens, MRP1 expression levels do not differ substantially from those determined in adjacent healthy tissue, although decreased MRP1 expression has been reported in a small proportion of samples analyzed [133]. The study of GAC-derived cell lines reveals a great variability in MRP1 expression, which inversely correlates with the sensitivity of these cells to anticancer drugs, such as etoposide, vincristine, epirubicin, doxorubicin, and vinblastine [135]. MRP1 has been proposed as a marker of chemoresistance in GAC, mainly associated with acquired resistance to cisplatin, although this drug is not believed to be a substrate of MRP1. Following treatment with anticancer platinum-based regimens, increased chemoresistance associated with higher MRP1 expression has been observed in both GAC patients and the cisplatin-resistant GAC-derived cell line KATOIII/DDP [119]. In addition, recent studies suggest that long non-coding RNAs (lncRNAs) are involved in the acquisition of the MDR phenotype, a process in which increased MRP1 expression is involved [136,137].
5.3. MRP2 and MRP3 in Gastric Adenocarcinoma
The relevance of MRP2 and MRP3 in developing the MDR phenotype in GAC cells is likely minor and null, respectively. MRP2 expression is inversely correlated with differentiation, being higher in poorly differentiated gastric tumors [138]. In GAC-derived cell lines, MRP2 expression varies from low to moderate [139,140].
Besides, MRP3 has not been detected at the protein level in healthy stomachs and GAC [44,132]. The same occurs in GAC-derived cells, except for SNU601 cells with acquired resistance to cisplatin, in which an increased MRP3 expression has been found [141].
5.4. MRP4 and MRP5 in Gastric Adenocarcinoma
MRP4 is abundantly expressed in healthy gastric mucosa and GAC [122,132]. In GAC biopsies, the MRP4 expression was significantly higher than in normal gastric tissue [142]. Furthermore, MRP4 levels are upregulated in several gastric cell lines, especially in those that have developed chemoresistance [120]. The inhibition of MRP4 expression in these cells by siRNA increases the sensitivity to 5-FU through cell cycle arrest and activation of apoptosis via BCL2/BAX [120]. Similarly, increased MRP4 expression has been described in the cisplatin-resistant cell line SGC7901, whose sensitivity to this drug was enhanced after ABCC4 silencing using siRNA [121]. MRP4 has also been associated with reduced GAC response to dasatinib [122].
Scarce information on MRP5 expression in GAC is available. Although MRP5 is known to transport 5-FU, there is no data on the relationship between this pump and the response of GAC to 5-FU.
5.5. BCRP in Gastric Adenocarcinoma
Immunohistochemical analysis of BCRP shows homogeneous and intense staining in the plasma membrane and intracellular compartment of untreated GAC [143]. In addition, in vitro studies have revealed increased ABCG2 mRNA levels in GAC cells exposed to cisplatin [144]. In GAC patients, elevated BCRP levels in tumor samples obtained before chemotherapy were associated with shorter OS [123]. Furthermore, after treatment with cisplatin and 5-FU, the residual cells have elevated expression of hedgehog (Hg) target genes GLI1 and GLI2, suggesting activation of Hg signaling. This pathway is a crucial regulator for putative cancer stem cells. Thus, enhanced GLI1/GLI2 expression is accompanied by BCRP upregulation, which is associated with decreased OS and increased risk of cancer relapse [124]. In addition, GLI2 knockdown sensitized GAC cells to 5-FU treatment. Because of the possible role of BCRP in GAC chemoresistance, identifying novel BCRP inhibitors able to enhance the response to anticancer drugs transported by this pump could be helpful. For example, genistein-induced inhibition of BCRP expression in GAC-derived cells is associated with increased sensitivity to 5-FU and cisplatin [145]. Furthermore, using ribozymes in GAC cells to reduce BCRP mRNA increased sensitivity to BCRP substrates [146].
6. Pancreatic Ductal Adenocarcinoma
Pancreatic ductal adenocarcinoma (PDAC) is another aggressive malignancy affecting the digestive system. The number of deaths caused by this cancer per year worldwide is close to 470,000, i.e., 4.7% of deaths due to cancer. The standard-of-care therapy for PDAC patients consists of 5-FU, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) [147], although some patients receive gemcitabine alone or combined with nab-paclitaxel or erlotinib. The benefit of chemotherapy is minimal because PDAC is characterized by its high chemoresistance, due in part to the elevated activity of ABC pumps accounting for the reduction of intracellular concentration of these drugs [148] (Table 6).
Table 6.
Role of ABC proteins in chemoresistance of pancreatic ductal adenocarcinoma (PDAC).
| Change | Drugs Affected | Impact | Ref. |
|---|---|---|---|
| MDR1 upregulation | Gemcitabine | Decreased cell sensitivity in vitro | [149,150] |
| MRP1 upregulation | 5-FU | Decreased cell sensitivity in vitro | [151,152] |
| MRP1 downregulation | Gemcitabine | Increased cell sensitivity in vitro | [153] |
| MRP2 upregulation | Gemcitabine | Reduced OS | [154] |
| MRP2 downregulation | Gemcitabine | Increased cell sensitivity in vitro | [79,155] |
| MRP3 upregulation | 5-FU | Decreased cell sensitivity in vitro | [156,157] |
| MRP4 upregulation | 5-FU | Decreased cell sensitivity in vitro | [156] |
| MRP5 upregulation | 5-FU | Decreased cell sensitivity in vitro | [158] |
| Gemcitabine | Decreased cell sensitivity in vitro | [159,160] | |
| BCRP upregulation | Gemcitabine | Worse prognosis | [161] |
5-FU, 5-Fluorouracil; BCRP, breast cancer resistance protein; MDR, multidrug resistance protein; MRP, multidrug resistant-associated protein; OS, overall survival.
6.1. MDR1 in Pancreatic Ductal Adenocarcinoma
As in other epithelial cells, MDR1 is expressed in the apical membrane of epithelial cells lining the pancreatic ducts [23], where it participates in the transport of components of the pancreatic secretion. Immunohistochemical analysis revealed that MDR1 expression is higher in non-treated PDAC than in healthy ducts [162,163]. Intense positive staining was observed in 52 out of 71 PDAC analyzed, and this was associated with a better prognosis in patients who did not receive chemotherapy [163]. MDR1 expression in PDAC-derived cell lines is a heterogeneous feature, ranging from undetectable or low to high [149,164,165]. The abundance of ABCB1 mRNA has been associated with resistance to gemcitabine [149] and taxanes, but not to 5-FU [166]. Gemcitabine-resistant pancreatic cell lines established by dose-escalation of the drug showed stemness characteristics and MDR1 overexpression [167].
6.2. MRP1 in Pancreatic Ductal Adenocarcinoma
MRP1 is expressed in fibroblasts but not in pancreatic acinar/ductal cells and PDAC [168]. Although MRP1 mRNA expression was high in 32 PDAC specimens compared to adjacent non-tumor tissue, minimal contribution to the poor response to treatment was attributed to this transporter [169]. However, more recent studies have demonstrated that MRP1 overexpression is associated with 5-FU [151] and gemcitabine resistance [152,170] in PDAC cells in vitro. Interestingly, molecular communication between cancer-associated fibroblasts (CAFs) and PDAC cells has been suggested as the mechanism triggering MRP1 downregulation and, consequently, reduced gemcitabine resistance in PDAC cells [153].
6.3. MRP2 in Pancreatic Ductal Adenocarcinoma
Immunohistochemical analyses have supported that MRP2 is not expressed in healthy exocrine pancreatic tissue [138,168]. However, there is controversy regarding the expression of this pump in PDAC, because although it was not detected by some groups [168], other studies have detected its presence in 91% of 67 tumor samples from patients who had not previously received any treatment [161]. Different research detected intracellular and plasma membrane staining of MRP2 in 77.5% of 40 tumor samples analyzed [171]. Patients bearing the MRP2 variant Gly40Ala showed a weak association with survival and tumor response to gemcitabine therapy and, surprisingly, this association diminished in patients receiving gemcitabine/cisplatin plus radiotherapy [154]. Regarding human pancreatic cell lines, one study described a low abundance of MRP2 mRNA in some PDAC-derived cells, but protein expression by Western blot was not found in any of the seven cell lines tested [156]. Another study reported moderate MRP2 mRNA expression in five cell lines and induction of its expression in cisplatin-resistant cells [171]. Besides, indirect downregulation of MRP2 gene expression was suggested to induce gemcitabine sensitivity in PDAC cell lines [155].
6.4. MRP3 in Pancreatic Ductal Adenocarcinoma
MRP3 is expressed in the basolateral membrane of normal pancreatic acinar/ductal cells and PDAC, with higher mRNA levels in more differentiated tumors [168]. Increased expression of MRP3 was associated with lower survival of PDAC patients and it was suggested to play an essential role in tumor growth in vivo [157]. MRP3 expression is variable among PDAC-derived cell lines and was upregulated both at mRNA and protein levels [156,157] in an established 5-FU resistant cell line [156], but not in gemcitabine-resistant cells [160].
6.5. MRP4 in Pancreatic Ductal Adenocarcinoma
MRP4 is localized in the plasma membrane of both ductal and acinar pancreatic cells and PDAC cells [168,172]. Still, there is controversy regarding the expression of this export pump in PDCA compared with normal ductal cells. Thus, one study reported similar expression in PDAC and healthy tissue, but another found higher expression in PDAC [168,172]. Variable mRNA and protein MRP4 levels in seven PDAC-derived cell lines have been reported. Upregulation of MRP4 in response to increased intracellular cAMP levels [173] and in 5-FU resistant cells [156] has been described.
6.6. MRP5 and MRP6 in Pancreatic Ductal Adenocarcinoma
MRP5 is also localized in the basolateral membrane of the duct and acinar cells and the plasma membrane of PDAC cells [168]. MRP5 mRNA levels were higher in PDAC than in normal tissue and were not associated with tumor grade or stage [168]. Several studies using PDAC-derived cell lines have shown that ABCC5 mRNA levels in these cells correlated significantly with their refractoriness to 5-FU [158]. Exposure of PDAC cells to gemcitabine or 5-FU induced MRP5 upregulation, which was associated with enhanced resistance to these anticancer agents [169,171,174].
The information regarding MRP6 expression in PDAC is scarce. The abundance of ABCC6 mRNA in healthy pancreas and PDAC specimens obtained before chemotherapy was very low [168].
6.7. BCRP in Pancreatic Ductal Adenocarcinoma
The abundance of ABCG2 mRNA in healthy pancreatic tissue is low. In contrast, although with marked interindividual variability, considerable ABCG2 mRNA levels have been found in PDAC samples from 31 patients undergoing partial pancreatectomy [168]. Heterogeneity in BCRP expression was confirmed by immunohistochemistry, which revealed positive staining in 73% of 65 PDAC samples analyzed, as well as an association between high BCRP expression, early recurrence, and poor survival of patients treated with adjuvant gemcitabine-based chemotherapy [161].
7. Colorectal Carcinoma
The available drug therapy to treat colorectal carcinoma (CRC) provides limited beneficial effects in patients with advanced disease [175]. Thus, close to 940,000 people die from CRC each year, which represents 9.4% of deaths due to cancer. Both conventional chemotherapy, based mainly on 5-FU and other pyrimidine analogs (e.g., capecitabine, trifluridine, and tipiracil), platinum agents (e.g., oxaliplatin), and irinotecan, and targeted therapy, based on TKIs (e.g., regorafenib), and monoclonal antibodies against TKRs (e.g., aflibercept, bevacizumab, cetuximab, panitumumab, and ramucirumab) are used [175]. Immunotherapy has recently been approved to treat CRC. The role of ABC proteins in CRC chemoresistance is commented below (Table 7).
Table 7.
Role of ABC proteins in chemoresistance of colorectal carcinoma (CRC).
| Change | Drugs Affected | Impact | Ref. |
|---|---|---|---|
| MDR1 upregulation | Doxorubicin, vincristine | Decreased cell sensitivity in vitro | [174] |
| ABCB5 upregulation | 5-FU | Worse response | [176] |
| MRP1 upregulation | 5-FU, oxaliplatin | Decreased cell sensitivity in vitro | [177,178] |
| Irinotecan | Worse response | [179] | |
| MRP1 variant rs17501011 | 5-FU, irinotecan | Reduced OS | [180] |
| MRP2 upregulation | Cisplatin | Worse response | [181] |
| Cisplatin | Decreased cell sensitivity in vitro | [182] | |
| MRP3 upregulation | Etoposide, oxaliplatin | Decreased cell sensitivity in vitro | [183,184] |
| MRP4 variant rs3742106 | 5-FU | Worse response | [185] |
| MRP5 upregulation | 5-FU, doxorubicin, cisplatin, oxaliplatin | Decreased cell sensitivity in vitro | [104] |
| BCRP downregulation | 5-FU, oxaliplatin | Increased cell sensitivity in vitro | [186] |
5-FU, 5-Fluorouracil; BCRP, breast cancer resistance protein; MDR, multidrug resistance protein; MRP, multidrug resistant-associated protein; OS, overall survival.
7.1. MDR1 in Colorectal Carcinoma
MDR1 is highly expressed in the colon mucosa [23]. The abundance of ABCB1 mRNA is increased in most CRCs, being significantly higher in well-differentiated than in poorly differentiated tumors [187] regardless of tumor location and size [188]. Immunohistochemical analysis of CRC biopsies also revealed a high proportion of MDR1 positivity among analyzed tumors [188]. Nevertheless, MDR1 is believed to play a minor role in CRC resistance to pharmacological treatment based on 5-FU, platinum derivatives, irinotecan, and its active metabolites because these drugs are not transported by MDR1 [189,190]. However, this pump contributes to the CRC intrinsic resistance to anthracyclines, Vinca alkaloids, epipodophyllotoxins, and taxanes, all of which are MDR1 substrates [191]. Unlike in tumors, in CRC-derived cell lines, MDR1 expression is higher in poorly differentiated than in more differentiated ones. Moreover, their MDR1 expression levels parallel the resistance to its drug substrates, such as anthracyclines and vincristine [174].
7.2. MRP1 in Colorectal Carcinoma
The role of MRP1 in CRC chemoresistance is controversial. Several studies have reported the lack of association [190,192], whereas others have observed a link between TNM staging and differentiation grade and enhanced MRP1 expression in CRC biopsies, revealed by positive immunohistochemical staining [193]. Moreover, in vitro testing has demonstrated that MRP1 is associated with resistance to oxaliplatin, 5-FU, and bevacizumab [177,178,194]. Besides, the detection of MRP1 in circulating tumor cells in patients with CRC has recently been suggested as a biomarker of irinotecan resistance [179,195]. Furthermore, the presence of the intron variants ABCC1 rs17501011 (c.49-20550G>A), CES1 rs9921399 (c.52+538A>G), UGT1A rs1113193 (c.855+41929G>A for UGT1A8 and c.855+23114G>A for UGT1A10) was related to lower OS in FOLFIRI-treated CRC patients [180].
7.3. MRP2 in Colorectal Carcinoma
High levels of MRP2 mRNA have been found in CRC compared with tissue from non-tumor surrounding mucosa and healthy individuals. Elevated MRP2 expression is considered one of the most critical mechanisms of chemoresistance, leading to the failure of CRC treatment based on cisplatin or oxaliplatin [181,196]. In several CRC-derived cell lines, MRP2 is constitutively expressed, their sensitivity to cisplatin being dependent on their ABCC2 mRNA levels [182], which correlate with reduced intracellular cisplatin accumulation [197]. Long-term exposure of these cells to cisplatin results in a marked stimulation of the expression of several ABC proteins, particularly MRP2 [198,199]. In addition, when cisplatin-sensitive cells were stably transfected with ABCC2 cDNA, they acquired drug resistance [77], which was reversed by incubation with probenecid, an MRP2 inhibitor [197].
7.4. MRP3 in Colorectal Carcinoma
The role of MRP3 in the response of CRC to chemotherapy seems irrelevant. Indeed, in surgically resected CRC, no relationship between MRP3 expression and the sensitivity to anticancer agents, such as doxorubicin, mitomycin C, cisplatin, 5-FU, etoposide, and camptothecin derivatives, has been found [181]. Moreover, MRP3 mRNA levels in CRC and colorectal polyps compared with non-tumor tissues are decreased or unchanged [181,200]. However, upregulation of MRP3 in CRC-derived cell lines contributed to resistance to oxaliplatin [183] and etoposide [184]. In addition, short-term exposure in vitro to cisplatin [199] or oxaliplatin and 5-FU [201] results in enhanced MRP3 expression.
7.5. MRP4 in Colorectal Carcinoma
Compared with healthy tissue, MRP4 expression is higher in human CRC, colorectal polyps, and CRC cell lines [202]. Despite the ability of MRP4 to transport 5-FU and irinotecan, there is no strong evidence linking MRP4 expression to the lack of response of CRC patients to these drugs. The rs3742106 variant of ABCC4 (c.*38A>C/T) affects the binding of miR-3190-5p to ABCC4 3′-UTR, which reduces its expression [185]. Thus, in patients with enhanced expression of this MRP4 variant, 5-FU concentrations in tumor cells are reduced [185]. It should be considered that CRC is a tumor with a marked inflammatory microenvironment. In this context, MRP4 plays a role in regulating the levels of prostaglandin E2 (PGE2), the most abundant COX-2-derived pro-carcinogenic prostaglandin in the CRC microenvironment [203]. Some authors have investigated the effect of celecoxib, a COX-2 inhibitor, in combination with irinotecan and oxaliplatin against CRC. However, trials have not shown an advantageous effect of this combination, which may be due to the fact that celecoxib induces MRP4 upregulation [204].
7.6. MRP5 in Colorectal Carcinoma
Genetic variants of ABCC5 (along with those of the ABCG1 and SLCO1B1) have been associated with the gastrointestinal toxicity of its substrate irinotecan [205]. Its administration should be avoided in combination with celecoxib because this drug increases MRP5 expression [204]. Experiments with cells transfected with MRP5 show increased efflux of 5-FU, adefovir, and purine analogs [104,206]. These studies also demonstrated that, in addition to 5-FU, MRP5 also confers resistance in CRC to several anticancer agents, including methotrexate, pemetrexed, doxorubicin, and the platinum-containing drugs cisplatin and oxaliplatin [104]. However, the role of MRP5 expression in CRC chemoresistance in the clinical setting has not been established.
7.7. BCRP in Colorectal Carcinoma
Compared with paired non-tumor tissue, a significantly lower abundance of ABCG2 mRNA was determined in CRC biopsies obtained before the patients received antitumor therapy [198]. However, BCRP is highly expressed in CD133-positive cells from human CRC. These are thought to be putative cancer stem-like cells with an associated lack of response, leading to tumor recurrence and metastasis [186,207]. High BCRP levels have been found in CRC-derived mitoxantrone- and cisplatin-resistant cell lines [199,208]. Downregulation of this efflux pump significantly enhances the efficacy of 5-FU and oxaliplatin in vitro and in vivo [186].
7.8. Other ABC Pumps in Colorectal Carcinoma
Regarding the role of other ABCs in the MDR phenotype of CRC, ABCB5 has been proposed as a marker of refractoriness to 5-FU treatment in these patients. This was due to the high levels of expression of this protein that was found in patients who did not respond to 5-FU-based chemotherapy regimens. Interestingly, the relationship between ABCB5 overexpression and 5-FU resistance was confirmed in a CRC xenograft model that had undergone 5-FU monotherapy [176].
8. Conclusions
Although there is still some confusion about the actual relevance of ABC pumps in the response of hepatobiliary, pancreatic, and gastrointestinal cancers to pharmacological treatment, there is a clear consensus on the need to consider them in future strategies to improve the efficacy of classical and newer drugs. The first step in this direction is to correctly identify the members of this superfamily of proteins expressed in each type of cancer (Figure 1). However, the existence of interindividual variability makes it necessary to define the MOC pattern on a tumor-by-tumor basis, even in a dynamic way, because it can vary during tumor progression and in response to chemotherapy. One important conclusion of this descriptive review is that each type of cancer has its own major characteristics defining its ABC expression pattern, which should be refined by deeper individual analyses and considered before starting any pharmacological treatment to get the highest probability of success in each patient. This information would also be required to develop novel strategies for sensitization.
Abbreviations
5-FU, 5-fluorouracil; BCRP, breast cancer resistance protein; BTC, biliary tract cancer; CAF, cancer-associated fibroblast; CCA, cholangiocarcinoma; CRC, colorectal carcinoma; eCCA, extrahepatic CCA; GAC, gastric adenocarcinoma; GBC, gallbladder cancer; HB, hepatoblastoma; HCC, hepatocellular carcinoma; iCCA, intrahepatic CCA; LCSC, liver cancer stem cells; MDR, multidrug resistance protein; MRP, multidrug resistant-associated protein; OS, overall survival; PDCA, pancreatic ductal adenocarcinoma; TACE, transarterial chemoembolization; TKI, tyrosine kinase inhibitor; TKR, tyrosine kinase receptor.
Author Contributions
All authors contributed equally to the elaboration of this review article. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
This study was funded by the CIBERehd (EHD15PI05/2016) and “Fondo de Investigaciones Sanitarias, Instituto de Salud Carlos III”, Spain (PI19/00819, and PI20/00189, co-funded by European Regional Development Fund/European Social Fund, “Investing in your future”); Spanish Ministry of Science and Innovation (MCIN/AEI/10.13039/501100011033, PID2020-119164RB-I00); “Junta de Castilla y Leon” (SA074P20); AECC Scientific Foundation (2017/2020), Spain; “Proyectos de Investigación. Modalidad C2”, University of Salamanca (18.K137/463AC01 and 18.K140/463AC01); “Centro Internacional sobre el Envejecimiento” (OLD-HEPAMARKER, 0348_CIE_6_E), Spain, V Beca de Investigación Carmen Delgado/Miguel Pérez-Mateo, Spain, and Fundación University of Salamanca, Spain (PC-TCUE18-20_051); Fundació Marato TV3 (Ref. 201916-31). “Avvio alla ricerca 2021”, Sapienza University grant, Italy (prot. AR22117A8682DF8B). Juan Cordoba Fellowship 2021 (Spanish association fot the study of the liver, AEEH). C.C.L. was supported by a predoctoral scholarship (FPU) funded by the Ministry of Science, Innovation and Universities, Spain. S.O.R. was supported by a postdoctoral contract funded by the “Junta de Castilla y Leon” (SA074P20), Spain. S.D.G. was supported by “Enrico and Enrica Sovena” Foundation (Italy).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marin J.J.G., Perez-Silva L., Macias R.I.R., Asensio M., Peleteiro-Vigil A., Sanchez-Martin A., Cives-Losada C., Sanchon-Sanchez P., Sanchez De Blas B., Herraez E., et al. Molecular bases of mechanisms accounting for drug resistance in gastric adenocarcinoma. Cancers. 2020;12:2116. doi: 10.3390/cancers12082116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marin J.J.G., Macias R.I.R., Monte M.J., Romero M.R., Asensio M., Sanchez-Martin A., Cives-Losada C., Temprano A.G., Espinosa-Escudero R., Reviejo M., et al. Molecular bases of drug resistance in hepatocellular carcinoma. Cancers. 2020;12:1663. doi: 10.3390/cancers12061663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marin J.J.G., Sanchon-Sanchez P., Cives-Losada C., Del Carmen S., Gonzalez-Santiago J.M., Monte M.J., Macias R.I.R. Novel pharmacological options in the treatment of cholangiocarcinoma: Mechanisms of resistance. Cancers. 2021;13:2358. doi: 10.3390/cancers13102358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dean M., Moitra K., Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Hum. Mutat. 2022. in press . [DOI] [PMC free article] [PubMed]
- 5.Pavlikova L., Seres M., Breier A., Sulova Z. The roles of microRNAs in cancer multidrug resistance. Cancers. 2022;14:1090. doi: 10.3390/cancers14041090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Giacomo S., Briz O., Monte M.J., Sanchez-Vicente L., Abete L., Lozano E., Mazzanti G., Di Sotto A., Marin J.J.G. Chemosensitization of hepatocellular carcinoma cells to sorafenib by beta-caryophyllene oxide-induced inhibition of ABC export pumps. Arch. Toxicol. 2019;93:623–634. doi: 10.1007/s00204-019-02395-9. [DOI] [PubMed] [Google Scholar]
- 7.Marin J.J.G., Romero M.R., Herraez E., Asensio M., Ortiz-Rivero S., Sanchez-Martin A., Fabris L., Briz O. Mechanisms of pharmacoresistance in hepatocellular carcinoma: New drugs but old problems. Semin. Liver Dis. 2022;42:87–103. doi: 10.1055/s-0041-1735631. [DOI] [PubMed] [Google Scholar]
- 8.Gao B., Yang F.M., Yu Z.T., Li R., Xie F., Chen J., Luo H.J., Zhang J.C. Relationship between the expression of MDR1 in hepatocellular cancer and its biological behaviors. Int. J. Clin. Exp. Pathol. 2015;8:6995–7001. [PMC free article] [PubMed] [Google Scholar]
- 9.Estevinho M.M., Fernandes C., Silva J.C., Gomes A.C., Afecto E., Correia J., Carvalho J. Role of ATP-binding cassette transporters in sorafenib therapy for hepatocellular carcinoma: An overview. Curr. Drug Targets. 2022;23:21–32. doi: 10.2174/1389450122666210412125018. [DOI] [PubMed] [Google Scholar]
- 10.Asghar U., Meyer T. Are there opportunities for chemotherapy in the treatment of hepatocellular cancer? J. Hepatol. 2012;56:686–695. doi: 10.1016/j.jhep.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 11.Tandia M., Mhiri A., Paule B., Saffroy R., Cailliez V., Noe G., Farinotti R., Bonhomme-Faivre L. Correlation between clinical response to sorafenib in hepatocellular carcinoma treatment and polymorphisms of P-glycoprotein (ABCB1) and of breast cancer resistance protein (ABCG2): Monocentric study. Cancer Chemother. Pharm. 2017;79:759–766. doi: 10.1007/s00280-017-3268-y. [DOI] [PubMed] [Google Scholar]
- 12.Cheung S.T., Cheung P.F., Cheng C.K., Wong N.C., Fan S.T. Granulin-epithelin precursor and ATP-dependent binding cassette (ABC)B5 regulate liver cancer cell chemoresistance. Gastroenterology. 2011;140:344–355. doi: 10.1053/j.gastro.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 13.Chang Y.S., Su C.W., Chen S.C., Chen Y.Y., Liang Y.J., Wu J.C. Upregulation of USP22 and ABCC1 during sorafenib treatment of hepatocellular carcinoma contribute to development of resistance. Cells. 2022;11:634. doi: 10.3390/cells11040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibayama Y., Nakano K., Maeda H., Taguchi M., Ikeda R., Sugawara M., Iseki K., Takeda Y., Yamada K. Multidrug resistance protein 2 implicates anticancer drug-resistance to sorafenib. Biol. Pharm. Bull. 2011;34:433–435. doi: 10.1248/bpb.34.433. [DOI] [PubMed] [Google Scholar]
- 15.Tomonari T., Takeishi S., Taniguchi T., Tanaka T., Tanaka H., Fujimoto S., Kimura T., Okamoto K., Miyamoto H., Muguruma N., et al. MRP3 as a novel resistance factor for sorafenib in hepatocellular carcinoma. Oncotarget. 2016;7:7207–7215. doi: 10.18632/oncotarget.6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wakamatsu T., Nakahashi Y., Hachimine D., Seki T., Okazaki K. The combination of glycyrrhizin and lamivudine can reverse the cisplatin resistance in hepatocellular carcinoma cells through inhibition of multidrug resistance-associated proteins. Int. J. Oncol. 2007;31:1465–1472. doi: 10.3892/ijo.31.6.1465. [DOI] [PubMed] [Google Scholar]
- 17.Rius M., Nies A.T., Hummel-Eisenbeiss J., Jedlitschky G., Keppler D. Cotransport of reduced glutathione with bile salts by MRP4 (ABCC4) localized to the basolateral hepatocyte membrane. Hepatology. 2003;38:374–384. doi: 10.1053/jhep.2003.50331. [DOI] [PubMed] [Google Scholar]
- 18.Borel F., Han R., Visser A., Petry H., van Deventer S.J., Jansen P.L., Konstantinova P., Reseau Centre de Ressources Biologiques Foie F. Adenosine triphosphate-binding cassette transporter genes up-regulation in untreated hepatocellular carcinoma is mediated by cellular microRNAs. Hepatology. 2012;55:821–832. doi: 10.1002/hep.24682. [DOI] [PubMed] [Google Scholar]
- 19.Wang X.B., Wang S.S., Zhang Q.F., Liu M., Li H.L., Liu Y., Wang J.N., Zheng F., Guo L.Y., Xiang J.Z. Inhibition of tetramethylpyrazine on P-gp, MRP2, MRP3 and MRP5 in multidrug resistant human hepatocellular carcinoma cells. Oncol. Rep. 2010;23:211–215. [PubMed] [Google Scholar]
- 20.Hu C., Li H., Li J., Zhu Z., Yin S., Hao X., Yao M., Zheng S., Gu J. Analysis of ABCG2 expression and side population identifies intrinsic drug efflux in the HCC cell line MHCC-97L and its modulation by Akt signaling. Carcinogenesis. 2008;29:2289–2297. doi: 10.1093/carcin/bgn223. [DOI] [PubMed] [Google Scholar]
- 21.Huang W.C., Hsieh Y.L., Hung C.M., Chien P.H., Chien Y.F., Chen L.C., Tu C.Y., Chen C.H., Hsu S.C., Lin Y.M., et al. BCRP/ABCG2 inhibition sensitizes hepatocellular carcinoma cells to sorafenib. PLoS ONE. 2013;8:e83627. doi: 10.1371/journal.pone.0083627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fung S.W., Cheung P.F., Yip C.W., Ng L.W., Cheung T.T., Chong C.C., Lee C., Lai P.B., Chan A.W., Tsao G.S., et al. The ATP-binding cassette transporter ABCF1 is a hepatic oncofetal protein that promotes chemoresistance, EMT and cancer stemness in hepatocellular carcinoma. Cancer Lett. 2019;457:98–109. doi: 10.1016/j.canlet.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Thiebaut F., Tsuruo T., Hamada H., Gottesman M.M., Pastan I., Willingham M.C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc. Natl. Acad. Sci. USA. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chenivesse X., Franco D., Brechot C. MDR1 (multidrug resistance) gene expression in human primary liver cancer and cirrhosis. J. Hepatol. 1993;18:168–172. doi: 10.1016/S0168-8278(05)80243-0. [DOI] [PubMed] [Google Scholar]
- 25.Ng I.O., Liu C.L., Fan S.T., Ng M. Expression of P-glycoprotein in hepatocellular carcinoma. A determinant of chemotherapy response. Am. J. Clin. Pathol. 2000;113:355–363. doi: 10.1309/AC1M-4TY4-U0TN-EN7T. [DOI] [PubMed] [Google Scholar]
- 26.Minemura M., Tanimura H., Tabor E. Overexpression of multidrug resistance genes MDR1 and cMOAT in human hepatocellular carcinoma and hepatoblastoma cell lines. Int. J. Oncol. 1999;15:559–563. doi: 10.3892/ijo.15.3.559. [DOI] [PubMed] [Google Scholar]
- 27.Kort A., Durmus S., Sparidans R.W., Wagenaar E., Beijnen J.H., Schinkel A.H. Brain and testis accumulation of regorafenib is restricted by Breast Cancer Resistance Protein (BCRP/ABCG2) and P-glycoprotein (P-GP/ABCB1) Pharm. Res. 2015;32:2205–2216. doi: 10.1007/s11095-014-1609-7. [DOI] [PubMed] [Google Scholar]
- 28.Shumaker R.C., Aluri J., Fan J., Martinez G., Thompson G.A., Ren M. Effect of rifampicin on the pharmacokinetics of lenvatinib in healthy adults. Clin. Drug Investig. 2014;34:651–659. doi: 10.1007/s40261-014-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keppler D. Drug Transporters. Handbook of Experimental Pharmacology. Springer; Berlin/Heidelberg, Germany: 2011. Multidrug resistance proteins (MRPs, ABCCs): Importance for pathophysiology and drug therapy; pp. 299–323. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann K., Shibo L., Xiao Z., Longerich T., Buchler M.W., Schemmer P. Correlation of gene expression of ATP-binding cassette protein and tyrosine kinase signaling pathway in patients with hepatocellular carcinoma. Anticancer Res. 2011;31:3883–3890. [PubMed] [Google Scholar]
- 31.Bonin S., Pascolo L., Croce L.S., Stanta G., Tiribelli C. Gene expression of ABC proteins in hepatocellular carcinoma, perineoplastic tissue, and liver diseases. Mol. Med. 2002;8:318–325. doi: 10.1007/BF03402158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nies A.T., Konig J., Pfannschmidt M., Klar E., Hofmann W.J., Keppler D. Expression of the multidrug resistance proteins MRP2 and MRP3 in human hepatocellular carcinoma. Int. J. Cancer. 2001;94:492–499. doi: 10.1002/ijc.1498. [DOI] [PubMed] [Google Scholar]
- 33.He S.M., Li R., Kanwar J.R., Zhou S.F. Structural and functional properties of human multidrug resistance protein 1 (MRP1/ABCC1) Curr. Med. Chem. 2011;18:439–481. doi: 10.2174/092986711794839197. [DOI] [PubMed] [Google Scholar]
- 34.Nies A.T., Keppler D. The apical conjugate efflux pump ABCC2 (MRP2) Pflug. Arch. 2007;453:643–659. doi: 10.1007/s00424-006-0109-y. [DOI] [PubMed] [Google Scholar]
- 35.Zollner G., Wagner M., Fickert P., Silbert D., Fuchsbichler A., Zatloukal K., Denk H., Trauner M. Hepatobiliary transporter expression in human hepatocellular carcinoma. Liver Int. 2005;25:367–379. doi: 10.1111/j.1478-3231.2005.01033.x. [DOI] [PubMed] [Google Scholar]
- 36.Vasilyeva A., Durmus S., Li L., Wagenaar E., Hu S., Gibson A.A., Panetta J.C., Mani S., Sparreboom A., Baker S.D., et al. Hepatocellular shuttling and recirculation of sorafenib-glucuronide is dependent on Abcc2, Abcc3, and Oatp1a/1b. Cancer Res. 2015;75:2729–2736. doi: 10.1158/0008-5472.CAN-15-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohya H., Shibayama Y., Ogura J., Narumi K., Kobayashi M., Iseki K. Regorafenib is transported by the organic anion transporter 1B1 and the multidrug resistance protein 2. Biol. Pharm. Bull. 2015;38:582–586. doi: 10.1248/bpb.b14-00740. [DOI] [PubMed] [Google Scholar]
- 38.Lacy S., Hsu B., Miles D., Aftab D., Wang R., Nguyen L. Metabolism and disposition of cabozantinib in healthy male volunteers and pharmacologic characterization of its major metabolites. Drug Metab. Dispos. 2015;43:1190–1207. doi: 10.1124/dmd.115.063610. [DOI] [PubMed] [Google Scholar]
- 39.Ozeki T., Nagahama M., Fujita K., Suzuki A., Sugino K., Ito K., Miura M. Influence of CYP3A4/5 and ABC transporter polymorphisms on lenvatinib plasma trough concentrations in Japanese patients with thyroid cancer. Sci. Rep. 2019;9:5404. doi: 10.1038/s41598-019-41820-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cirqueira C.S., Felipe-Silva A.S., Wakamatsu A., Marins L.V., Rocha E.C., de Mello E.S., Alves V.A.F. Immunohistochemical assessment of the expression of biliary transportation proteins MRP2 and MRP3 in hepatocellular carcinoma and in cholangiocarcinoma. Pathol. Oncol. Res. 2019;25:1363–1371. doi: 10.1007/s12253-018-0386-8. [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Becerra P., Vaquero J., Romero M.R., Lozano E., Anadon C., Macias R.I., Serrano M.A., Grane-Boladeras N., Munoz-Bellvis L., Alvarez L., et al. No correlation between the expression of FXR and genes involved in multidrug resistance phenotype of primary liver tumors. Mol. Pharm. 2012;9:1693–1704. doi: 10.1021/mp300028a. [DOI] [PubMed] [Google Scholar]
- 42.Jouan E., Le Vee M., Denizot C., Parmentier Y., Fardel O. Drug transporter expression and activity in human hepatoma HuH-7 cells. Pharmaceutics. 2016;9:3. doi: 10.3390/pharmaceutics9010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Vee M., Noel G., Jouan E., Stieger B., Fardel O. Polarized expression of drug transporters in differentiated human hepatoma HepaRG cells. Toxicol. In Vitro. 2013;27:1979–1986. doi: 10.1016/j.tiv.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Kool M., de Haas M., Scheffer G.L., Scheper R.J., van Eijk M.J., Juijn J.A., Baas F., Borst P. Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res. 1997;57:3537–3547. [PubMed] [Google Scholar]
- 45.Moustafa M.A., Ogino D., Nishimura M., Ueda N., Naito S., Furukawa M., Uchida T., Ikai I., Sawada H., Fukumoto M. Comparative analysis of ATP-binding cassette (ABC) transporter gene expression levels in peripheral blood leukocytes and in liver with hepatocellular carcinoma. Cancer Sci. 2004;95:530–536. doi: 10.1111/j.1349-7006.2004.tb03244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jedlitschky G., Burchell B., Keppler D. The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J. Biol. Chem. 2000;275:30069–30074. doi: 10.1074/jbc.M005463200. [DOI] [PubMed] [Google Scholar]
- 47.Jilek J.L., Tu M.J., Zhang C., Yu A.M. Pharmacokinetic and pharmacodynamic factors contribute to synergism between Let-7c-5p and 5-fluorouracil in inhibiting hepatocellular carcinoma cell viability. Drug Metab. Dispos. 2020;48:1257–1263. doi: 10.1124/dmd.120.000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maliepaard M., Scheffer G.L., Faneyte I.F., van Gastelen M.A., Pijnenborg A.C., Schinkel A.H., van De Vijver M.J., Scheper R.J., Schellens J.H. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–3464. [PubMed] [Google Scholar]
- 49.Sukowati C.H., Rosso N., Pascut D., Anfuso B., Torre G., Francalanci P., Croce L.S., Tiribelli C. Gene and functional up-regulation of the BCRP/ABCG2 transporter in hepatocellular carcinoma. BMC Gastroenterol. 2012;12:160. doi: 10.1186/1471-230X-12-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y.L., Chen P.M., Lin P.Y., Hsiau Y.T., Chu P.Y. ABCG2 overexpression confers poor outcomes in hepatocellular carcinoma of elderly patients. Anticancer Res. 2016;36:2983–2988. [PubMed] [Google Scholar]
- 51.Xie Z.Y., Liu M.S., Zhang C., Cai P.C., Xiao Z.H., Wang F.F. Aspirin enhances the sensitivity of hepatocellular carcinoma side population cells to doxorubicin via miR-491/ABCG2. Biosci. Rep. 2018;38:BSR20180854. doi: 10.1042/BSR20180854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang G., Wang Z., Luo W., Jiao H., Wu J., Jiang C. Expression of potential cancer stem cell marker ABCG2 is associated with malignant behaviors of hepatocellular carcinoma. Gastroenterol. Res. Pract. 2013;2013:782581. doi: 10.1155/2013/782581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsunedomi R., Yoshimura K., Kimura Y., Nishiyama M., Fujiwara N., Matsukuma S., Kanekiyo S., Matsui H., Shindo Y., Watanabe Y., et al. Elevated expression of RAB3B plays important roles in chemoresistance and metastatic potential of hepatoma cells. BMC Cancer. 2022;22:260. doi: 10.1186/s12885-022-09370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Namisaki T., Schaeffeler E., Fukui H., Yoshiji H., Nakajima Y., Fritz P., Schwab M., Nies A.T. Differential expression of drug uptake and efflux transporters in Japanese patients with hepatocellular carcinoma. Drug Metab. Dispos. 2014;42:2033–2040. doi: 10.1124/dmd.114.059832. [DOI] [PubMed] [Google Scholar]
- 55.Agarwal S., Sane R., Ohlfest J.R., Elmquist W.F. The role of the breast cancer resistance protein (ABCG2) in the distribution of sorafenib to the brain. J. Pharm. Exp. 2011;336:223–233. doi: 10.1124/jpet.110.175034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Briz O., Perez-Silva L., Al-Abdulla R., Abete L., Reviejo M., Romero M.R., Marin J.J.G. What “The Cancer Genome Atlas” database tells us about the role of ATP-binding cassette (ABC) proteins in chemoresistance to anticancer drugs. Expert Opin. Drug Metab. Toxicol. 2019;15:577–593. doi: 10.1080/17425255.2019.1631285. [DOI] [PubMed] [Google Scholar]
- 57.Cheung P.F., Cheung T.T., Yip C.W., Ng L.W., Fung S.W., Lo C.M., Fan S.T., Cheung S.T. Hepatic cancer stem cell marker granulin-epithelin precursor and beta-catenin expression associate with recurrence in hepatocellular carcinoma. Oncotarget. 2016;7:21644–21657. doi: 10.18632/oncotarget.7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marin J.J.G., Prete M.G., Lamarca A., Tavolari S., Landa-Magdalena A., Brandi G., Segatto O., Vogel A., Macias R.I.R., Rodrigues P.M., et al. Current and novel therapeutic opportunities for systemic therapy in biliary cancer. Br. J. Cancer. 2020;123:1047–1059. doi: 10.1038/s41416-020-0987-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marin J.J.G., Macias R.I.R., Cives-Losada C., Peleteiro-Vigil A., Herraez E., Lozano E. Plasma membrane transporters as biomarkers and molecular targets in cholangiocarcinoma. Cells. 2020;9:498. doi: 10.3390/cells9020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang H., Zhan M., Xu S.W., Chen W., Long M.M., Shi Y.H., Liu Q., Mohan M., Wang J. miR-218-5p restores sensitivity to gemcitabine through PRKCE/MDR1 axis in gallbladder cancer. Cell Death Dis. 2017;8:e2770. doi: 10.1038/cddis.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wattanawongdon W., Hahnvajanawong C., Namwat N., Kanchanawat S., Boonmars T., Jearanaikoon P., Leelayuwat C., Techasen A., Seubwai W. Establishment and characterization of gemcitabine-resistant human cholangiocarcinoma cell lines with multidrug resistance and enhanced invasiveness. Int. J. Oncol. 2015;47:398–410. doi: 10.3892/ijo.2015.3019. [DOI] [PubMed] [Google Scholar]
- 62.Wu W.R., Zhang R., Shi X.D., Zhu M.S., Xu L.B., Zeng H., Liu C. Notch1 is overexpressed in human intrahepatic cholangiocarcinoma and is associated with its proliferation, invasiveness and sensitivity to 5-fluorouracil in vitro. Oncol. Rep. 2014;31:2515–2524. doi: 10.3892/or.2014.3123. [DOI] [PubMed] [Google Scholar]
- 63.Srimunta U., Sawanyawisuth K., Kraiklang R., Pairojkul C., Puapairoj A., Titipungul T., Hahnvajanawong C., Tassaneeyakul W., Wongkham C., Wongkham S., et al. High expression of ABCC1 indicates poor prognosis in intrahepatic cholangiocarcinoma. Asian Pac. J. Cancer Prev. 2012;13:125–130. [PubMed] [Google Scholar]
- 64.Zhan M., Zhao X., Wang H., Chen W., Xu S., Wang W., Shen H., Huang S., Wang J. miR-145 sensitizes gallbladder cancer to cisplatin by regulating multidrug resistance associated protein 1. Tumour Biol. 2016;37:10553–10562. doi: 10.1007/s13277-016-4957-6. [DOI] [PubMed] [Google Scholar]
- 65.Lozano E., Asensio M., Perez-Silva L., Banales J.M., Briz O., Marin J.J.G. MRP3-mediated chemoresistance in cholangiocarcinoma: Target for chemosensitization through restoring SOX17 expression. Hepatology. 2020;72:949–964. doi: 10.1002/hep.31088. [DOI] [PubMed] [Google Scholar]
- 66.Yang J., Sontag D., Gong Y., Minuk G.Y. Enhanced gemcitabine cytotoxicity with knockdown of multidrug resistance protein genes in human cholangiocarcinoma cell lines. J. Gastroenterol. Hepatol. 2021;36:1103–1109. doi: 10.1111/jgh.15289. [DOI] [PubMed] [Google Scholar]
- 67.Sribenja S., Natthasirikul N., Vaeteewoottacharn K., Sawanyawisuth K., Wongkham C., Jearanaikoon P., Wongkham S. Thymosin beta10 as a predictive biomarker of response to 5-fluorouracil chemotherapy in cholangiocarcinoma. Ann. Hepatol. 2016;15:577–585. [PubMed] [Google Scholar]
- 68.Pavelic Z.P., Reising J., Pavelic L., Kelley D.J., Stambrook P.J., Gluckman J.L. Detection of P-glycoprotein with four monoclonal antibodies in normal and tumor tissues. Arch. Otolaryngol. Head Neck Surg. 1993;119:753–757. doi: 10.1001/archotol.1993.01880190049010. [DOI] [PubMed] [Google Scholar]
- 69.Wang B.L., Zhai H.Y., Chen B.Y., Zhai S.P., Yang H.Y., Chen X.P., Zhao W.T., Meng L. Clinical relationship between MDR1 gene and gallbladder cancer. Hepatobiliary Pancreat. Dis. Int. 2004;3:296–299. [PubMed] [Google Scholar]
- 70.Marin J.J.G., Lozano E., Briz O., Al-Abdulla R., Serrano M.A., Macias R.I.R. Molecular bases of chemoresistance in cholangiocarcinoma. Curr. Drug Targets. 2017;18:889–900. doi: 10.2174/1389450116666150223121508. [DOI] [PubMed] [Google Scholar]
- 71.Rost D., Konig J., Weiss G., Klar E., Stremmel W., Keppler D. Expression and localization of the multidrug resistance proteins MRP2 and MRP3 in human gallbladder epithelia. Gastroenterology. 2001;121:1203–1208. doi: 10.1053/gast.2001.28648. [DOI] [PubMed] [Google Scholar]
- 72.Pangestu N.S., Chueakwon P., Talabnin K., Khiaowichit J., Talabnin C. RNF43 overexpression attenuates the Wnt/beta-catenin signalling pathway to suppress tumour progression in cholangiocarcinoma. Oncol. Lett. 2021;22:846. doi: 10.3892/ol.2021.13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rau S., Autschbach F., Riedel H.D., Konig J., Kulaksiz H., Stiehl A., Riemann J.F., Rost D. Expression of the multidrug resistance proteins MRP2 and MRP3 in human cholangiocellular carcinomas. Eur. J. Clin. Investig. 2008;38:134–142. doi: 10.1111/j.1365-2362.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 74.Kim H.S., Kim N.C., Chae K.H., Kim G., Park W.S., Park Y.K., Kim Y.W. Expression of multidrug resistance-associated protein 2 in human gallbladder carcinoma. BioMed Res. Int. 2013;2013:527534. doi: 10.1155/2013/527534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tepsiri N., Chaturat L., Sripa B., Namwat W., Wongkham S., Bhudhisawasdi V., Tassaneeyakul W. Drug sensitivity and drug resistance profiles of human intrahepatic cholangiocarcinoma cell lines. World J. Gastroenterol. 2005;11:2748–2753. doi: 10.3748/wjg.v11.i18.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Samatiwat P., Prawan A., Senggunprai L., Kukongviriyapan U., Kukongviriyapan V. Nrf2 inhibition sensitizes cholangiocarcinoma cells to cytotoxic and antiproliferative activities of chemotherapeutic agents. Tumour Biol. 2016;37:11495–11507. doi: 10.1007/s13277-016-5015-0. [DOI] [PubMed] [Google Scholar]
- 77.Cui Y., Konig J., Buchholz J.K., Spring H., Leier I., Keppler D. Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, MRP2, permanently expressed in human and canine cells. Mol. Pharm. 1999;55:929–937. [PubMed] [Google Scholar]
- 78.Myint K., Biswas R., Li Y., Jong N., Jamieson S., Liu J., Han C., Squire C., Merien F., Lu J., et al. Identification of MRP2 as a targetable factor limiting oxaliplatin accumulation and response in gastrointestinal cancer. Sci. Rep. 2019;9:2245. doi: 10.1038/s41598-019-38667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu J., Hu G., Gong Y., Yu Q., He B., Li W., He Z., Hao W., He Z., Liu Y. Silencing of TRPM8 inhibits aggressive tumor phenotypes and enhances gemcitabine sensitivity in pancreatic cancer. Pancreatology. 2018;18:935–944. doi: 10.1016/j.pan.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 80.Scheffer G.L., Kool M., de Haas M., de Vree J.M., Pijnenborg A.C., Bosman D.K., Elferink R.P., van der Valk P., Borst P., Scheper R.J. Tissue distribution and induction of human multidrug resistant protein 3. Lab. Investig. 2002;82:193–201. doi: 10.1038/labinvest.3780411. [DOI] [PubMed] [Google Scholar]
- 81.Wang J., Zhang M., Zhang L., Cai H., Zhou S., Zhang J., Wang Y. Correlation of Nrf2, HO-1, and MRP3 in gallbladder cancer and their relationships to clinicopathologic features and survival. J. Surg. Res. 2010;164:e99–e105. doi: 10.1016/j.jss.2010.05.058. [DOI] [PubMed] [Google Scholar]
- 82.Kurzawski M., Dziedziejko V., Post M., Wojcicki M., Urasinska E., Mietkiewski J., Drozdzik M. Expression of genes involved in xenobiotic metabolism and transport in end-stage liver disease: Up-regulation of ABCC4 and CYP1B1. Pharm. Rep. 2012;64:927–939. doi: 10.1016/S1734-1140(12)70888-5. [DOI] [PubMed] [Google Scholar]
- 83.Kool M., van der Linden M., de Haas M., Baas F., Borst P. Expression of human MRP6, a homologue of the multidrug resistance protein gene MRP1, in tissues and cancer cells. Cancer Res. 1999;59:175–182. [PubMed] [Google Scholar]
- 84.Vander Borght S., Libbrecht L., Katoonizadeh A., van Pelt J., Cassiman D., Nevens F., Van Lommel A., Petersen B.E., Fevery J., Jansen P.L., et al. Breast cancer resistance protein (BCRP/ABCG2) is expressed by progenitor cells/reactive ductules and hepatocytes and its expression pattern is influenced by disease etiology and species type: Possible functional consequences. J. Histochem. Cytochem. 2006;54:1051–1059. doi: 10.1369/jhc.5A6912.2006. [DOI] [PubMed] [Google Scholar]
- 85.Aust S., Obrist P., Jaeger W., Klimpfinger M., Tucek G., Wrba F., Penner E., Thalhammer T. Subcellular localization of the ABCG2 transporter in normal and malignant human gallbladder epithelium. Lab. Investig. 2004;84:1024–1036. doi: 10.1038/labinvest.3700127. [DOI] [PubMed] [Google Scholar]
- 86.Meyers R.L., Maibach R., Hiyama E., Haberle B., Krailo M., Rangaswami A., Aronson D.C., Malogolowkin M.H., Perilongo G., von Schweinitz D., et al. Risk-stratified staging in paediatric hepatoblastoma: A unified analysis from the Children’s Hepatic tumors International Collaboration. Lancet Oncol. 2017;18:122–131. doi: 10.1016/S1470-2045(16)30598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Ioris M., Brugieres L., Zimmermann A., Keeling J., Brock P., Maibach R., Pritchard J., Shafford L., Zsiros J., Czaudzerna P., et al. Hepatoblastoma with a low serum alpha-fetoprotein level at diagnosis: The SIOPEL group experience. Eur. J. Cancer. 2008;44:545–550. doi: 10.1016/j.ejca.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 88.Marin J.J.G., Cives-Losada C., Asensio M., Lozano E., Briz O., Macias R.I.R. Mechanisms of anticancer drug resistance in hepatoblastoma. Cancers. 2019;11:407. doi: 10.3390/cancers11030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen Y.B., Yan M.L., Gong J.P., Xia R.P., Liu L.X., Li N., Lu S.C., Zhang J.G., Zeng D.B., Xie J.G., et al. Establishment of hepatocellular carcinoma multidrug resistant monoclone cell line HepG2/mdr1. Chin. Med. J. 2007;120:703–707. doi: 10.1097/00029330-200704020-00017. [DOI] [PubMed] [Google Scholar]
- 90.Korita P.V., Wakai T., Shirai Y., Matsuda Y., Sakata J., Takamura M., Yano M., Sanpei A., Aoyagi Y., Hatakeyama K., et al. Multidrug resistance-associated protein 2 determines the efficacy of cisplatin in patients with hepatocellular carcinoma. Oncol. Rep. 2010;23:965–972. doi: 10.3892/or_00000721. [DOI] [PubMed] [Google Scholar]
- 91.Oue T., Yoneda A., Uehara S., Yamanaka H., Fukuzawa M. Increased expression of multidrug resistance-associated genes after chemotherapy in pediatric solid malignancies. J. Pediatr. Surg. 2009;44:377–380. doi: 10.1016/j.jpedsurg.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 92.Warmann S., Hunger M., Teichmann B., Flemming P., Gratz K.F., Fuchs J. The role of the MDR1 gene in the development of multidrug resistance in human hepatoblastoma: Clinical course and in vivo model. Cancer. 2002;95:1795–1801. doi: 10.1002/cncr.10858. [DOI] [PubMed] [Google Scholar]
- 93.Liu T., Wei R., Zhang Y., Chen W., Liu H. Association between NF-kappaB expression and drug resistance of liver cancer. Oncol. Lett. 2019;17:1030–1034. doi: 10.3892/ol.2018.9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bader P., Fuchs J., Wenderoth M., von Schweinitz D., Niethammer D., Beck J.F. Altered expression of resistance associated genes in hepatoblastoma xenografts incorporated into mice following treatment with adriamycin or cisplatin. Anticancer Res. 1998;18:3127–3132. [PubMed] [Google Scholar]
- 95.Warmann S.W., Heitmann H., Teichmann B., Gratz K.F., Ruck P., Hunger M., Fuchs J. Effects of P-glycoprotein modulation on the chemotherapy of xenotransplanted human hepatoblastoma. Pediatr. Hematol. Oncol. 2005;22:373–386. doi: 10.1080/08880010590964192. [DOI] [PubMed] [Google Scholar]
- 96.Pascolo L., Fernetti C., Pirulli D., Bogoni S., Garcia-Mediavilla M.V., Spano A., Puzzer D., Tiribelli C., Amoroso A., Crovella S. Detection of MRP1 mRNA in human tumors and tumor cell lines by in situ RT-PCR. Biochem. Biophys. Res. Commun. 2000;275:466–471. doi: 10.1006/bbrc.2000.3339. [DOI] [PubMed] [Google Scholar]
- 97.Eicher C., Dewerth A., Kirchner B., Warmann S.W., Fuchs J., Armeanu-Ebinger S. Development of a drug resistance model for hepatoblastoma. Int. J. Oncol. 2011;38:447–454. doi: 10.3892/ijo.2010.860. [DOI] [PubMed] [Google Scholar]
- 98.Vander Borght S., van Pelt J., van Malenstein H., Cassiman D., Renard M., Verslype C., Libbrecht L., Roskams T.A. Up-regulation of breast cancer resistance protein expression in hepatoblastoma following chemotherapy: A study in patients and in vitro. Hepatol. Res. 2008;38:1112–1121. doi: 10.1111/j.1872-034X.2008.00381.x. [DOI] [PubMed] [Google Scholar]
- 99.Su Z., Liu G., Fang T., Wang Y., Zhang H., Yang S., Wei J., Lv Z., Tan L., Liu J. Silencing MRP1-4 genes by RNA interference enhances sensitivity of human hepatoma cells to chemotherapy. Am. J. Transl. Res. 2016;8:2790–2802. [PMC free article] [PubMed] [Google Scholar]
- 100.Liu X.Y., Liu S.P., Jiang J., Zhang X., Zhang T. Inhibition of the JNK signaling pathway increases sensitivity of hepatocellular carcinoma cells to cisplatin by down-regulating expression of P-glycoprotein. Eur. Rev. Med. Pharmacol. Sci. 2016;20:1098–1108. [PubMed] [Google Scholar]
- 101.Schrenk D., Baus P.R., Ermel N., Klein C., Vorderstemann B., Kauffmann H.M. Up-regulation of transporters of the MRP family by drugs and toxins. Toxicol. Lett. 2001;120:51–57. doi: 10.1016/S0378-4274(01)00306-X. [DOI] [PubMed] [Google Scholar]
- 102.Lagas J.S., Fan L., Wagenaar E., Vlaming M.L., van Tellingen O., Beijnen J.H., Schinkel A.H. P-glycoprotein (P-gp/Abcb1), Abcc2, and Abcc3 determine the pharmacokinetics of etoposide. Clin. Cancer Res. 2010;16:130–140. doi: 10.1158/1078-0432.CCR-09-1321. [DOI] [PubMed] [Google Scholar]
- 103.Liu W., Song H., Chen Q., Xu C., Zhang W., Liu Y., Wang B., Xu D., Lu M., Yang D., et al. Multidrug resistance protein 4 is a critical protein associated with the antiviral efficacy of nucleos(t)ide analogues. Liver Int. 2016;36:1284–1294. doi: 10.1111/liv.13104. [DOI] [PubMed] [Google Scholar]
- 104.Pratt S., Shepard R.L., Kandasamy R.A., Johnston P.A., Perry W., 3rd, Dantzig A.H. The multidrug resistance protein 5 (ABCC5) confers resistance to 5-fluorouracil and transports its monophosphorylated metabolites. Mol. Cancer. 2005;4:855–863. doi: 10.1158/1535-7163.MCT-04-0291. [DOI] [PubMed] [Google Scholar]
- 105.Borst P., de Wolf C., van de Wetering K. Multidrug resistance-associated proteins 3, 4, and 5. Pflug. Arch. 2007;453:661–673. doi: 10.1007/s00424-006-0054-9. [DOI] [PubMed] [Google Scholar]
- 106.Lee G., Piquette-Miller M. Influence of IL-6 on MDR and MRP-mediated multidrug resistance in human hepatoma cells. Can. J. Physiol. Pharm. 2001;79:876–884. doi: 10.1139/y01-071. [DOI] [PubMed] [Google Scholar]
- 107.Belinsky M.G., Chen Z.S., Shchaveleva I., Zeng H., Kruh G.D. Characterization of the drug resistance and transport properties of multidrug resistance protein 6 (MRP6, ABCC6) Cancer Res. 2002;62:6172–6177. [PubMed] [Google Scholar]
- 108.Hopper-Borge E., Xu X., Shen T., Shi Z., Chen Z.S., Kruh G.D. Human multidrug resistance protein 7 (ABCC10) is a resistance factor for nucleoside analogues and epothilone B. Cancer Res. 2009;69:178–184. doi: 10.1158/0008-5472.CAN-08-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Joshi S.S., Badgwell B.D. Current treatment and recent progress in gastric cancer. CA A Cancer J. Clin. 2021;71:264–279. doi: 10.3322/caac.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Al-Batran S.E., Homann N., Pauligk C., Goetze T.O., Meiler J., Kasper S., Kopp H.G., Mayer F., Haag G.M., Luley K., et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet. 2019;393:1948–1957. doi: 10.1016/S0140-6736(18)32557-1. [DOI] [PubMed] [Google Scholar]
- 111.Elimova E., Janjigian Y.Y., Mulcahy M., Catenacci D.V., Blum M.A., Almhanna K., Hecht J.R., Ajani J.A. It is time to stop using epirubicin to treat any patient with gastroesophageal adenocarcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017;35:475–477. doi: 10.1200/JCO.2016.69.7276. [DOI] [PubMed] [Google Scholar]
- 112.Ivey A., Pratt H., Boone B.A. Molecular pathogenesis and emerging targets of gastric adenocarcinoma. J. Surg. Oncol. 2022;125:1079–1095. doi: 10.1002/jso.26874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marin J.J., Romero M.R., Blazquez A.G., Herraez E., Keck E., Briz O. Importance and limitations of chemotherapy among the available treatments for gastrointestinal tumours. Anti-Cancer Agents Med. Chem. 2009;9:162–184. doi: 10.2174/187152009787313828. [DOI] [PubMed] [Google Scholar]
- 114.Zhai J., Shen J., Xie G., Wu J., He M., Gao L., Zhang Y., Yao X., Shen L. Cancer-associated fibroblasts-derived IL-8 mediates resistance to cisplatin in human gastric cancer. Cancer Lett. 2019;454:37–43. doi: 10.1016/j.canlet.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 115.Danza K., Silvestris N., Simone G., Signorile M., Saragoni L., Brunetti O., Monti M., Mazzotta A., De Summa S., Mangia A., et al. Role of miR-27a, miR-181a and miR-20b in gastric cancer hypoxia-induced chemoresistance. Cancer Biol. Ther. 2016;17:400–406. doi: 10.1080/15384047.2016.1139244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu X., Zheng Y., Han B., Dong X. Long noncoding RNA BLACAT1 modulates ABCB1 to promote oxaliplatin resistance of gastric cancer via sponging miR-361. Biomed. Pharmacother. 2018;99:832–838. doi: 10.1016/j.biopha.2018.01.130. [DOI] [PubMed] [Google Scholar]
- 117.Mo D., Fang H., Niu K., Liu J., Wu M., Li S., Zhu T., Aleskandarany M.A., Arora A., Lobo D.N., et al. Human helicase RECQL4 drives cisplatin resistance in gastric cancer by activating an AKT-YB1-MDR1 signaling pathway. Cancer Res. 2016;76:3057–3066. doi: 10.1158/0008-5472.CAN-15-2361. [DOI] [PubMed] [Google Scholar]
- 118.Felipe A.V., Oliveira J., Moraes A.A., Franca J.P., Silva T.D., Forones N.M. Reversal of multidrug resistance in an epirubicin-resistant gastric cancer cell subline. Asian Pac. J. Cancer Prev. 2018;19:1237–1242. doi: 10.22034/APJCP.2018.19.5.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wongsirisin P., Limpakan Yamada S., Yodkeeree S., Punfa W., Limtrakul P. Association of DNA repair and drug transporter in relation to chemosensitivity in primary culture of Thai gastric cancer patients. Biol. Pharm. Bull. 2018;41:360–367. doi: 10.1248/bpb.b17-00688. [DOI] [PubMed] [Google Scholar]
- 120.Zhang G., Wang Z., Qian F., Zhao C., Sun C. Silencing of the ABCC4 gene by RNA interference reverses multidrug resistance in human gastric cancer. Oncol. Rep. 2015;33:1147–1154. doi: 10.3892/or.2014.3702. [DOI] [PubMed] [Google Scholar]
- 121.Zhang Y.H., Wu Q., Xiao X.Y., Li D.W., Wang X.P. Silencing MRP4 by small interfering RNA reverses acquired DDP resistance of gastric cancer cell. Cancer Lett. 2010;291:76–82. doi: 10.1016/j.canlet.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 122.Furmanski B.D., Hu S., Fujita K.I., Li L., Gibson A.A., Janke L.J., Williams R.T., Schuetz J.D., Sparreboom A., Baker S.D. Contribution of ABCC4-mediated gastric transport to the absorption and efficacy of dasatinib. Clin. Cancer Res. 2013;19:4359–4370. doi: 10.1158/1078-0432.CCR-13-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang Q., Li K., Xu J.H., Zhao C.G., Gao Q., Wu B., Liu X.Y. Role of ABCG2 expression driven by cisplatin in platinum-containing chemotherapy for gastric cancer. World J. Gastroenterol. 2013;19:6630–6636. doi: 10.3748/wjg.v19.i39.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yu B., Gu D., Zhang X., Liu B., Xie J. The role of GLI2-ABCG2 signaling axis for 5Fu resistance in gastric cancer. J. Genet. Genom. 2017;44:375–383. doi: 10.1016/j.jgg.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fojo A.T., Ueda K., Slamon D.J., Poplack D.G., Gottesman M.M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc. Natl. Acad. Sci. USA. 1987;84:265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rocco A., Compare D., Liguori E., Cianflone A., Pirozzi G., Tirino V., Bertoni A., Santoriello M., Garbi C., D’Armiento M., et al. MDR1-P-glycoprotein behaves as an oncofetal protein that promotes cell survival in gastric cancer cells. Lab. Investig. 2012;92:1407–1418. doi: 10.1038/labinvest.2012.100. [DOI] [PubMed] [Google Scholar]
- 127.Gurel S., Yerci O., Filiz G., Dolar E., Yilmazlar T., Nak S.G., Gulten M., Zorluoglu A., Memik F. High expression of multidrug resistance-1 (MDR-1) and its relationship with multiple prognostic factors in gastric carcinomas in patients in Turkey. J. Int. Med. Res. 1999;27:79–84. doi: 10.1177/030006059902700204. [DOI] [PubMed] [Google Scholar]
- 128.Wallner J., Depisch D., Gsur A., Gotzl M., Haider K., Pirker R. MDR1 gene expression and its clinical relevance in primary gastric carcinomas. Cancer. 1993;71:667–671. doi: 10.1002/1097-0142(19930201)71:3<667::AID-CNCR2820710303>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 129.de Oliveira J., Felipe A.V., Neto R.A., Oshima C.T., de Souza Silva M., Forones N.M. Association between ABCB1 immunohistochemical expression and overall survival in gastric cancer patients. Asian Pac. J. Cancer Prev. 2014;15:6935–6938. doi: 10.7314/APJCP.2014.15.16.6935. [DOI] [PubMed] [Google Scholar]
- 130.Lage H. Molecular analysis of therapy resistance in gastric cancer. Dig. Dis. 2003;21:326–338. doi: 10.1159/000075356. [DOI] [PubMed] [Google Scholar]
- 131.Prime-Chapman H.M., Fearn R.A., Cooper A.E., Moore V., Hirst B.H. Differential multidrug resistance-associated protein 1 through 6 isoform expression and function in human intestinal epithelial Caco-2 cells. J. Pharm. Exp. 2004;311:476–484. doi: 10.1124/jpet.104.068775. [DOI] [PubMed] [Google Scholar]
- 132.Al-Abdulla R., Perez-Silva L., Lozano E., Macias R.I.R., Herraez E., Abad M., Segues N., Bujanda L., Briz O., Marin J.J.G. Sensitizing gastric adenocarcinoma to chemotherapy by pharmacological manipulation of drug transporters. Biochem. Pharm. 2020;171:113682. doi: 10.1016/j.bcp.2019.113682. [DOI] [PubMed] [Google Scholar]
- 133.Lacueva J., Perez-Ramos M., Soto J.L., Oliver I., Andrada E., Medrano J., Perez-Vazquez T., Arroyo A., Carrato A., Ferragut J.A., et al. Multidrug resistance-associated protein (MRP1) gene is strongly expressed in gastric carcinomas. Analysis by immunohistochemistry and real-time quantitative RT-PCR. Histopathology. 2005;46:389–395. doi: 10.1111/j.1365-2559.2005.02100.x. [DOI] [PubMed] [Google Scholar]
- 134.Alexander D., Yamamoto T., Kato S., Kasai S. Histopathological assessment of multidrug resistance in gastric cancer: Expression of P-glycoprotein, multidrug resistance-associated protein, and lung-resistance protein. Surg. Today. 1999;29:401–406. doi: 10.1007/BF02483030. [DOI] [PubMed] [Google Scholar]
- 135.Obuchi W., Ohtsuki S., Uchida Y., Ohmine K., Yamori T., Terasaki T. Identification of transporters associated with Etoposide sensitivity of stomach cancer cell lines and methotrexate sensitivity of breast cancer cell lines by quantitative targeted absolute proteomics. Mol. Pharm. 2013;83:490–500. doi: 10.1124/mol.112.081083. [DOI] [PubMed] [Google Scholar]
- 136.Zhang X., Bo P., Liu L., Zhang X., Li J. Overexpression of long non-coding RNA GHET1 promotes the development of multidrug resistance in gastric cancer cells. Biomed. Pharmacother. 2017;92:580–585. doi: 10.1016/j.biopha.2017.04.111. [DOI] [PubMed] [Google Scholar]
- 137.Xu Y.D., Shang J., Li M., Zhang Y.Y. LncRNA DANCR accelerates the development of multidrug resistance of gastric cancer. Eur. Rev. Med. Pharmacol. Sci. 2019;23:2794–2802. doi: 10.26355/eurrev_201904_17554. [DOI] [PubMed] [Google Scholar]
- 138.Sandusky G.E., Mintze K.S., Pratt S.E., Dantzig A.H. Expression of multidrug resistance-associated protein 2 (MRP2) in normal human tissues and carcinomas using tissue microarrays. Histopathology. 2002;41:65–74. doi: 10.1046/j.1365-2559.2002.01403.x. [DOI] [PubMed] [Google Scholar]
- 139.Narasaki F., Oka M., Nakano R., Ikeda K., Fukuda M., Nakamura T., Soda H., Nakagawa M., Kuwano M., Kohno S. Human canalicular multispecific organic anion transporter (cMOAT) is expressed in human lung, gastric, and colorectal cancer cells. Biochem. Biophys. Res. Commun. 1997;240:606–611. doi: 10.1006/bbrc.1997.7703. [DOI] [PubMed] [Google Scholar]
- 140.Chen X., Zhang M., Liu L.X. The overexpression of multidrug resistance-associated proteins and gankyrin contribute to arsenic trioxide resistance in liver and gastric cancer cells. Oncol. Rep. 2009;22:73–80. [PubMed] [Google Scholar]
- 141.Gotovdorj T., Lee E., Lim Y., Cha E.J., Kwon D., Hong E., Kim Y., Oh M.Y. 2,3,7,8-Tetrachlorodibenzo-p-dioxin induced cell-specific drug transporters with acquired cisplatin resistance in cisplatin sensitive cancer cells. J. Korean Med. Sci. 2014;29:1188–1198. doi: 10.3346/jkms.2014.29.9.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wu Q., Li Z.S., Li D.W., Peng Z.H., Yang Z.R. [Relationship between the expression of hypoxia-inducible factor-1alpha and chemotherapy response in gastric carcinoma] Zhonghua Wei Chang Wai Ke Za Zhi = Chin. J. Gastrointest. Surg. 2009;12:498–501. [PubMed] [Google Scholar]
- 143.Diestra J.E., Scheffer G.L., Catala I., Maliepaard M., Schellens J.H., Scheper R.J., Germa-Lluch J.R., Izquierdo M.A. Frequent expression of the multi-drug resistance-associated protein BCRP/MXR/ABCP/ABCG2 in human tumours detected by the BXP-21 monoclonal antibody in paraffin-embedded material. J. Pathol. 2002;198:213–219. doi: 10.1002/path.1203. [DOI] [PubMed] [Google Scholar]
- 144.Zhan D., Ni T., Wang H., Lv M., Sunagawa M., Liu Y. Celastrol inhibits the proliferation and decreases drug resistance of cisplatin- resistant gastric cancer SGC7901/DDP cells. Anti-Cancer Agents Med. Chem. 2022;22:270–279. doi: 10.2174/1871520621666210528144006. [DOI] [PubMed] [Google Scholar]
- 145.Huang W., Wan C., Luo Q., Huang Z., Luo Q. Genistein-inhibited cancer stem cell-like properties and reduced chemoresistance of gastric cancer. Int. J. Mol. Sci. 2014;15:3432–3443. doi: 10.3390/ijms15033432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kowalski P., Stein U., Scheffer G.L., Lage H. Modulation of the atypical multidrug-resistant phenotype by a hammerhead ribozyme directed against the ABC transporter BCRP/MXR/ABCG2. Cancer Gene Ther. 2002;9:579–586. doi: 10.1038/sj.cgt.7700471. [DOI] [PubMed] [Google Scholar]
- 147.Conroy T., Desseigne F., Ychou M., Bouche O., Guimbaud R., Becouarn Y., Adenis A., Raoul J.L., Gourgou-Bourgade S., de la Fouchardiere C., et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 148.Quinonero F., Mesas C., Doello K., Cabeza L., Perazzoli G., Jimenez-Luna C., Rama A.R., Melguizo C., Prados J. The challenge of drug resistance in pancreatic ductal adenocarcinoma: A current overview. Cancer Biol. Med. 2019;16:688–699. doi: 10.20892/j.issn.2095-3941.2019.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen M., Xue X., Wang F., An Y., Tang D., Xu Y., Wang H., Yuan Z., Gao W., Wei J., et al. Expression and promoter methylation analysis of ATP-binding cassette genes in pancreatic cancer. Oncol. Rep. 2012;27:265–269. doi: 10.3892/or.2011.1475. [DOI] [PubMed] [Google Scholar]
- 150.Song B., Liu X.S., Rice S.J., Kuang S., Elzey B.D., Konieczny S.F., Ratliff T.L., Hazbun T., Chiorean E.G., Liu X. Plk1 phosphorylation of orc2 and hbo1 contributes to gemcitabine resistance in pancreatic cancer. Mol. Cancer. 2013;12:58–68. doi: 10.1158/1535-7163.MCT-12-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ouyang L., Liu R.D., Lei D.Q., Shang Q.C., Li H.F., Hu X.G., Zheng H., Jin G. MiR-499a-5p promotes 5-FU resistance and the cell proliferation and migration through activating PI3K/Akt signaling by targeting PTEN in pancreatic cancer. Ann. Transl. Med. 2021;9:1798. doi: 10.21037/atm-21-6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gu J., Huang W., Wang X., Zhang J., Tao T., Zheng Y., Liu S., Yang J., Chen Z.S., Cai C.Y., et al. Hsa-miR-3178/RhoB/PI3K/Akt, a novel signaling pathway regulates ABC transporters to reverse gemcitabine resistance in pancreatic cancer. Mol. Cancer. 2022;21:112. doi: 10.1186/s12943-022-01587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wei L., Lin Q., Lu Y., Li G., Huang L., Fu Z., Chen R., Zhou Q. Cancer-associated fibroblasts-mediated ATF4 expression promotes malignancy and gemcitabine resistance in pancreatic cancer via the TGF-beta1/SMAD2/3 pathway and ABCC1 transactivation. Cell Death Dis. 2021;12:334. doi: 10.1038/s41419-021-03574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tanaka M., Okazaki T., Suzuki H., Abbruzzese J.L., Li D. Association of multi-drug resistance gene polymorphisms with pancreatic cancer outcome. Cancer. 2011;117:744–751. doi: 10.1002/cncr.25510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Horiguchi S., Shiraha H., Nagahara T., Kataoka J., Iwamuro M., Matsubara M., Nishina S., Kato H., Takaki A., Nouso K., et al. Loss of runt-related transcription factor 3 induces gemcitabine resistance in pancreatic cancer. Mol. Oncol. 2013;7:840–849. doi: 10.1016/j.molonc.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hagmann W., Jesnowski R., Faissner R., Guo C., Lohr J.M. ATP-binding cassette C transporters in human pancreatic carcinoma cell lines. Upregulation in 5-fluorouracil-resistant cells. Pancreatology. 2009;9:136–144. doi: 10.1159/000178884. [DOI] [PubMed] [Google Scholar]
- 157.Adamska A., Ferro R., Lattanzio R., Capone E., Domenichini A., Damiani V., Chiorino G., Akkaya B.G., Linton K.J., De Laurenzi V., et al. ABCC3 is a novel target for the treatment of pancreatic cancer. Adv. Biol. Regul. 2019;73:100634. doi: 10.1016/j.jbior.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 158.Nambaru P.K., Hubner T., Kock K., Mews S., Grube M., Payen L., Guitton J., Sendler M., Jedlitschky G., Rimmbach C., et al. Drug efflux transporter multidrug resistance-associated protein 5 affects sensitivity of pancreatic cancer cell lines to the nucleoside anticancer drug 5-fluorouracil. Drug Metab. Dispos. 2011;39:132–139. doi: 10.1124/dmd.110.033613. [DOI] [PubMed] [Google Scholar]
- 159.Hagmann W., Faissner R., Schnolzer M., Lohr M., Jesnowski R. Membrane drug transporters and chemoresistance in human pancreatic carcinoma. Cancers. 2010;3:106–125. doi: 10.3390/cancers3010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hagmann W., Jesnowski R., Lohr J.M. Interdependence of gemcitabine treatment, transporter expression, and resistance in human pancreatic carcinoma cells. Neoplasia. 2010;12:740–747. doi: 10.1593/neo.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee S.H., Kim H., Hwang J.H., Lee H.S., Cho J.Y., Yoon Y.S., Han H.S. Breast cancer resistance protein expression is associated with early recurrence and decreased survival in resectable pancreatic cancer patients. Pathol. Int. 2012;62:167–175. doi: 10.1111/j.1440-1827.2011.02772.x. [DOI] [PubMed] [Google Scholar]
- 162.Lu Z., Kleeff J., Shrikhande S., Zimmermann T., Korc M., Friess H., Buchler M.W. Expression of the multidrug-resistance 1 (MDR1) gene and prognosis in human pancreatic cancer. Pancreas. 2000;21:240–247. doi: 10.1097/00006676-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 163.Suwa H., Ohshio G., Arao S., Imamura T., Yamaki K., Manabe T., Imamura M., Hiai H., Fukumoto M. Immunohistochemical localization of P-glycoprotein and expression of the multidrug resistance-1 gene in human pancreatic cancer: Relevance to indicator of better prognosis. Jpn. J. Cancer Res. 1996;87:641–649. doi: 10.1111/j.1349-7006.1996.tb00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Harpstrite S.E., Gu H., Natarajan R., Sharma V. Interrogation of multidrug resistance (MDR1) P-glycoprotein (ABCB1) expression in human pancreatic carcinoma cells: Correlation of 99mTc-Sestamibi uptake with western blot analysis. Nucl. Med. Commun. 2014;35:1067–1070. doi: 10.1097/MNM.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhao Y.P., Zhang L.Y., Liao Q., Guo J.C., Chen G., Li J.Y. Detection of multidrug resistant gene 1 in pancreatic cancer. Hepatobiliary Pancreat. Dis. Int. 2004;3:307–310. [PubMed] [Google Scholar]
- 166.Liu B., Staren E.D., Iwamura T., Appert H.E., Howard J.M. Mechanisms of taxotere-related drug resistance in pancreatic carcinoma. J. Surg. Res. 2001;99:179–186. doi: 10.1006/jsre.2001.6126. [DOI] [PubMed] [Google Scholar]
- 167.Hong S.P., Wen J., Bang S., Park S., Song S.Y. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int. J. Cancer. 2009;125:2323–2331. doi: 10.1002/ijc.24573. [DOI] [PubMed] [Google Scholar]
- 168.Konig J., Hartel M., Nies A.T., Martignoni M.E., Guo J., Buchler M.W., Friess H., Keppler D. Expression and localization of human multidrug resistance protein (ABCC) family members in pancreatic carcinoma. Int. J. Cancer. 2005;115:359–367. doi: 10.1002/ijc.20831. [DOI] [PubMed] [Google Scholar]
- 169.Mohelnikova-Duchonova B., Brynychova V., Oliverius M., Honsova E., Kala Z., Muckova K., Soucek P. Differences in transcript levels of ABC transporters between pancreatic adenocarcinoma and nonneoplastic tissues. Pancreas. 2013;42:707–716. doi: 10.1097/MPA.0b013e318279b861. [DOI] [PubMed] [Google Scholar]
- 170.Cao J., Yang J., Ramachandran V., Arumugam T., Deng D., Li Z., Xu L., Logsdon C.D. TM4SF1 promotes gemcitabine resistance of pancreatic cancer in vitro and in vivo. PLoS ONE. 2015;10:e0144969. doi: 10.1371/journal.pone.0144969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Noma B., Sasaki T., Fujimoto Y., Serikawa M., Kobayashi K., Inoue M., Itsuki H., Kamigaki M., Minami T., Chayama K. Expression of multidrug resistance-associated protein 2 is involved in chemotherapy resistance in human pancreatic cancer. Int. J. Oncol. 2008;33:1187–1194. doi: 10.3892/ijo_00000108. [DOI] [PubMed] [Google Scholar]
- 172.Zhang Z., Wang J., Shen B., Peng C., Zheng M. The ABCC4 gene is a promising target for pancreatic cancer therapy. Gene. 2012;491:194–199. doi: 10.1016/j.gene.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 173.Carozzo A., Diez F., Gomez N., Cabrera M., Shayo C., Davio C., Fernandez N. Dual role of cAMP in the transcriptional regulation of multidrug resistance-associated protein 4 (MRP4) in pancreatic adenocarcinoma cell lines. PLoS ONE. 2015;10:e0120651. doi: 10.1371/journal.pone.0120651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kramer R., Weber T.K., Morse B., Arceci R., Staniunas R., Steele G., Jr., Summerhayes I.C. Constitutive expression of multidrug resistance in human colorectal tumours and cell lines. Br. J. Cancer. 1993;67:959–968. doi: 10.1038/bjc.1993.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Marin J.J.G., Macias R.I.R., Monte M.J., Herraez E., Peleteiro-Vigil A., Blas B.S., Sanchon-Sanchez P., Temprano A.G., Espinosa-Escudero R.A., Lozano E., et al. Cellular mechanisms accounting for the refractoriness of colorectal carcinoma to pharmacological treatment. Cancers. 2020;12:2605. doi: 10.3390/cancers12092605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wilson B.J., Schatton T., Zhan Q., Gasser M., Ma J., Saab K.R., Schanche R., Waaga-Gasser A.M., Gold J.S., Huang Q., et al. ABCB5 identifies a therapy-refractory tumor cell population in colorectal cancer patients. Cancer Res. 2011;71:5307–5316. doi: 10.1158/0008-5472.CAN-11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cao D., Qin S., Mu Y., Zhong M. The role of MRP1 in the multidrug resistance of colorectal cancer. Oncol. Lett. 2017;13:2471–2476. doi: 10.3892/ol.2017.5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gao R., Fang C., Xu J., Tan H., Li P., Ma L. LncRNA CACS15 contributes to oxaliplatin resistance in colorectal cancer by positively regulating ABCC1 through sponging miR-145. Arch. Biochem. Biophys. 2019;663:183–191. doi: 10.1016/j.abb.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 179.Abdallah E.A., Fanelli M.F., Souza E.S.V., Machado Netto M.C., Gasparini Junior J.L., Araujo D.V., Ocea L.M., Buim M.E., Tariki M.S., Alves Vda S., et al. MRP1 expression in CTCs confers resistance to irinotecan-based chemotherapy in metastatic colorectal cancer. Int. J. Cancer. 2016;139:890–898. doi: 10.1002/ijc.30082. [DOI] [PubMed] [Google Scholar]
- 180.Labriet A., Levesque E., De Mattia E., Cecchin E., Jonker D., Couture F., Simonyan D., Buonadonna A., D’Andrea M., Villeneuve L., et al. Combination of germline variations associated with survival of folinic acid, fluorouracil and irinotecan-treated metastatic colorectal cancer patients. Pharmacogenomics. 2019;20:1179–1187. doi: 10.2217/pgs-2019-0091. [DOI] [PubMed] [Google Scholar]
- 181.Hinoshita E., Uchiumi T., Taguchi K., Kinukawa N., Tsuneyoshi M., Maehara Y., Sugimachi K., Kuwano M. Increased expression of an ATP-binding cassette superfamily transporter, multidrug resistance protein 2, in human colorectal carcinomas. Clin. Cancer Res. 2000;6:2401–2407. [PubMed] [Google Scholar]
- 182.Koike K., Kawabe T., Tanaka T., Toh S., Uchiumi T., Wada M., Akiyama S., Ono M., Kuwano M. A canalicular multispecific organic anion transporter (cMOAT) antisense cDNA enhances drug sensitivity in human hepatic cancer cells. Cancer Res. 1997;57:5475–5479. [PubMed] [Google Scholar]
- 183.Dong Y., Wang Z., Xie G.F., Li C., Zuo W.W., Meng G., Xu C.P., Li J.J. Pregnane X receptor is associated with unfavorable survival and induces chemotherapeutic resistance by transcriptional activating multidrug resistance-related protein 3 in colorectal cancer. Mol. Cancer. 2017;16:71. doi: 10.1186/s12943-017-0641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kobayashi M., Funayama R., Ohnuma S., Unno M., Nakayama K. Wnt-beta-catenin signaling regulates ABCC3 (MRP3) transporter expression in colorectal cancer. Cancer Sci. 2016;107:1776–1784. doi: 10.1111/cas.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chen Q., Meng F., Wang L., Mao Y., Zhou H., Hua D., Zhang H., Wang W. A polymorphism in ABCC4 is related to efficacy of 5-FU/capecitabine-based chemotherapy in colorectal cancer patients. Sci. Rep. 2017;7:7059. doi: 10.1038/s41598-017-07491-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ma L., Liu T., Jin Y., Wei J., Yang Y., Zhang H. ABCG2 is required for self-renewal and chemoresistance of CD133-positive human colorectal cancer cells. Tumour Biol. 2016;37:12889–12896. doi: 10.1007/s13277-016-5209-5. [DOI] [PubMed] [Google Scholar]
- 187.Mizoguchi T., Yamada K., Furukawa T., Hidaka K., Hisatsugu T., Shimazu H., Tsuruo T., Sumizawa T., Akiyama S. Expression of the MDR1 gene in human gastric and colorectal carcinomas. J. Natl. Cancer Inst. 1990;82:1679–1683. doi: 10.1093/jnci/82.21.1679. [DOI] [PubMed] [Google Scholar]
- 188.Pirker R., Wallner J., Gsur A., Gotzl M., Zochbauer S., Scheithauer W., Depisch D. MDR1 gene expression in primary colorectal carcinomas. Br. J. Cancer. 1993;68:691–694. doi: 10.1038/bjc.1993.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Iyer L., Ramirez J., Shepard D.R., Bingham C.M., Hossfeld D.K., Ratain M.J., Mayer U. Biliary transport of irinotecan and metabolites in normal and P-glycoprotein-deficient mice. Cancer Chemother. Pharm. 2002;49:336–341. doi: 10.1007/s00280-001-0420-4. [DOI] [PubMed] [Google Scholar]
- 190.Micsik T., Lorincz A., Mersich T., Baranyai Z., Besznyak I., Jr., Dede K., Zarand A., Jakab F., Szollosi L.K., Keri G., et al. Decreased functional activity of multidrug resistance protein in primary colorectal cancer. Diagn. Pathol. 2015;10:26. doi: 10.1186/s13000-015-0264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Efferth T., Konkimalla V.B., Wang Y.F., Sauerbrey A., Meinhardt S., Zintl F., Mattern J., Volm M. Prediction of broad spectrum resistance of tumors towards anticancer drugs. Clin. Cancer Res. 2008;14:2405–2412. doi: 10.1158/1078-0432.CCR-07-4525. [DOI] [PubMed] [Google Scholar]
- 192.Nishioka C., Sakaeda T., Nakamura T., Moriya Y., Okamura N., Tamura T., Nakahara T., Aoyama N., Kamigaki T., Ohno M., et al. MDR1, MRP1 and MRP2 genotypes and in vitro chemosensitivity in Japanese patients with colorectal adenocarcinomas. Kobe J. Med. Sci. 2004;50:181–188. [PubMed] [Google Scholar]
- 193.Yang J., Song P., Zhou G. A study on the correlations of MRP-1 expression with the pathogenesis and prognosis of colorectal cancer. J. BUON. 2019;24:84–90. [PubMed] [Google Scholar]
- 194.Huang W., Zhang H., Tian Y., Li Y., Li J., Zhong X., Yuan X. LncRNA SNHG11 enhances bevacizumab resistance in colorectal cancer by mediating miR-1207-5p/ABCC1 axis. Anti-Cancer Drugs. 2022;33:575–586. doi: 10.1097/CAD.0000000000001289. [DOI] [PubMed] [Google Scholar]
- 195.Silva V.S.E., Abdallah E.A., Brito A.B.C., Braun A.C., Tariki M.S., de Mello C.A.L., Calsavara V.F., Riechelmann R., Chinen L.T.D. Baseline and kinetic circulating tumor cell counts are prognostic factors in a prospective study of metastatic colorectal cancer. Diagnostics. 2021;11:502. doi: 10.3390/diagnostics11030502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang Z., Sun X., Feng Y., Liu X., Zhou L., Sui H., Ji Q., E Q., Chen J., Wu L., et al. Dihydromyricetin reverses MRP2-mediated MDR and enhances anticancer activity induced by oxaliplatin in colorectal cancer cells. Anti-Cancer Drugs. 2017;28:281–288. doi: 10.1097/CAD.0000000000000459. [DOI] [PubMed] [Google Scholar]
- 197.Monte M.J., Ballestero M.R., Briz O., Perez M.J., Marin J.J. Proapoptotic effect on normal and tumor intestinal cells of cytostatic drugs with enterohepatic organotropism. J. Pharm. Exp. 2005;315:24–35. doi: 10.1124/jpet.105.086165. [DOI] [PubMed] [Google Scholar]
- 198.Martinez-Becerra P., Monte I., Romero M.R., Serrano M.A., Vaquero J., Macias R.I., Del Rio A., Grane-Boladeras N., Jimenez F., San-Martin F.G., et al. Up-regulation of FXR isoforms is not required for stimulation of the expression of genes involved in the lack of response of colon cancer to chemotherapy. Pharm. Res. 2012;66:419–427. doi: 10.1016/j.phrs.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 199.Herraez E., Gonzalez-Sanchez E., Vaquero J., Romero M.R., Serrano M.A., Marin J.J., Briz O. Cisplatin-induced chemoresistance in colon cancer cells involves FXR-dependent and FXR-independent up-regulation of ABC proteins. Mol. Pharm. 2012;9:2565–2576. doi: 10.1021/mp300178a. [DOI] [PubMed] [Google Scholar]
- 200.Ballestero M.R., Monte M.J., Briz O., Jimenez F., Gonzalez-San Martin F., Marin J.J. Expression of transporters potentially involved in the targeting of cytostatic bile acid derivatives to colon cancer and polyps. Biochem. Pharm. 2006;72:729–738. doi: 10.1016/j.bcp.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 201.Park G.B., Chung Y.H., Kim D. 2-Deoxy-D-glucose suppresses the migration and reverses the drug resistance of colon cancer cells through ADAM expression regulation. Anti-Cancer Drugs. 2017;28:410–420. doi: 10.1097/CAD.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 202.Holla V.R., Backlund M.G., Yang P., Newman R.A., DuBois R.N. Regulation of prostaglandin transporters in colorectal neoplasia. Cancer Prev. Res. 2008;1:93–99. doi: 10.1158/1940-6207.CAPR-07-0009. [DOI] [PubMed] [Google Scholar]
- 203.Pereira C., Queiros S., Galaghar A., Sousa H., Pimentel-Nunes P., Brandao C., Moreira-Dias L., Medeiros R., Dinis-Ribeiro M. Genetic variability in key genes in prostaglandin E2 pathway (COX-2, HPGD, ABCC4 and SLCO2A1) and their involvement in colorectal cancer development. PLoS ONE. 2014;9:e92000. doi: 10.1371/journal.pone.0092000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gradilone A., Pulcinelli F.M., Lotti L.V., Trifiro E., Martino S., Gandini O., Gianni W., Frati L., Agliano A.M., Gazzaniga P. Celecoxib upregulates multidrug resistance proteins in colon cancer: Lack of synergy with standard chemotherapy. Curr. Cancer. Drug Targets. 2008;8:414–420. doi: 10.2174/156800908785133178. [DOI] [PubMed] [Google Scholar]
- 205.Di Martino M.T., Arbitrio M., Leone E., Guzzi P.H., Rotundo M.S., Ciliberto D., Tomaino V., Fabiani F., Talarico D., Sperlongano P., et al. Single nucleotide polymorphisms of ABCC5 and ABCG1 transporter genes correlate to irinotecan-associated gastrointestinal toxicity in colorectal cancer patients: A DMET microarray profiling study. Cancer Biol. Ther. 2011;12:780–787. doi: 10.4161/cbt.12.9.17781. [DOI] [PubMed] [Google Scholar]
- 206.Wijnholds J., Mol C.A., van Deemter L., de Haas M., Scheffer G.L., Baas F., Beijnen J.H., Scheper R.J., Hatse S., De Clercq E., et al. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc. Natl. Acad. Sci. USA. 2000;97:7476–7481. doi: 10.1073/pnas.120159197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chen K., Huang Y.H., Chen J.L. Understanding and targeting cancer stem cells: Therapeutic implications and challenges. Acta Pharm. Sin. 2013;34:732–740. doi: 10.1038/aps.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ross D.D., Yang W., Abruzzo L.V., Dalton W.S., Schneider E., Lage H., Dietel M., Greenberger L., Cole S.P., Doyle L.A. Atypical multidrug resistance: Breast cancer resistance protein messenger RNA expression in mitoxantrone-selected cell lines. J. Natl. Cancer Inst. 1999;91:429–433. doi: 10.1093/jnci/91.5.429. [DOI] [PubMed] [Google Scholar]