Abstract
Simple Summary
The resection margin status is one of the most relevant oncological factors in liver cancer surgery. Whether robotic liver surgery enhances R0 results in liver malignancies during minimally invasive liver surgery is not yet completely clear. We conducted a systematic review with meta-analysis to compare robotic and laparoscopic approaches in liver surgery with particular attention to the resection margin status in liver malignancies.
Abstract
Background: Robotic procedures are an integral part of modern liver surgery. However, the advantages of a robotic approach in comparison to the conventional laparoscopic approach are the subject of controversial debate. The aim of this systematic review and meta-analysis is to compare robotic and laparoscopic liver resection with particular attention to the resection margin status in malignant cases. Methods: A systematic literature search was performed using PubMed and Cochrane Library in accordance with the PRISMA guidelines. Only studies comparing robotic and laparoscopic liver resections were considered for this meta-analysis. Furthermore, the rate of the positive resection margin or R0 rate in malignant cases had to be clearly identifiable. We used fixed or random effects models according to heterogeneity. Results: Fourteen studies with a total number of 1530 cases were included in qualitative and quantitative synthesis. Malignancies were identified in 71.1% (n = 1088) of these cases. These included hepatocellular carcinoma, cholangiocarcinoma, colorectal liver metastases and other malignancies of the liver. Positive resection margins were noted in 24 cases (5.3%) in the robotic group and in 54 cases (8.6%) in the laparoscopic group (OR = 0.71; 95% CI (0.42–1.18); p = 0.18). Tumor size was significantly larger in the robotic group (MD = 6.92; 95% CI (2.93–10.91); p = 0.0007). The operation time was significantly longer in the robotic procedure (MD = 28.12; 95% CI (3.66–52.57); p = 0.02). There were no significant differences between the robotic and laparoscopic approaches regarding the intra-operative blood loss, length of hospital stay, overall and severe complications and conversion rate. Conclusion: Our meta-analysis showed no significant difference between the robotic and laparoscopic procedures regarding the resection margin status. Tumor size was significantly larger in the robotic group. However, randomized controlled trials with long-term follow-up are needed to demonstrate the benefits of robotics in liver surgery.
Keywords: liver surgery, robotic surgery, laparoscopic surgery, hepatectomy, resection margin, meta-analysis
1. Introduction
Robotic procedures are an integral part of modern liver surgery [1]. Various meta-analyses confirmed the comparability of robotic and laparoscopic approaches. With regard to the tumor-free resection margin, robotic and laparoscopic liver surgery show similar outcomes [2,3,4]. The resection margin status is one of the most important oncological parameters in liver cancer surgery [5]. A positive resection margin is an independent risk factor for recurrence-free and overall survival in patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma [6,7]. R1 resections of colorectal liver metastases in patients receiving perioperative chemotherapy were associated with significantly higher rates of intrahepatic and surgical margin recurrence [8].
Due to the earlier adoption of laparoscopic liver surgery, the number of laparoscopic cases is higher in many studies when compared to robotic liver surgery cases. Since a significant number of these surgeries were performed for benign indications, careful differentiation needs to be taken into account during statistical analysis of the R status. Otherwise, this could lead to a falsely lower percentage of positive resection margin rates.
In some studies and meta-analyses, no attention was paid to the accurate separation of the malignant and benign liver lesions when analyzing the R status of the resection margins. This resulted in inaccurate percentages of the R0 or R positive rates [9,10,11,12,13,14]. Moreover, several individual studies showed that robotic liver surgery achieved an R0 resection in 100% of the cases [15,16,17,18,19,20]. This gave us the idea to take a closer look at the previously published literature in order to systematically analyze the potential advantage of robotics with regard to tumor-free resection margins.
Stable three-dimensional visualization, absence of physiological tremor, higher freedom of movement, better ergonomics for the surgeon and the possibility of using a third arm are the advantages of robots compared to conventional laparoscopic surgery [21,22,23]. Perhaps these advantages of robotics are also beneficial in achieving R0 resection. Furthermore, the use of modern tools in minimally invasive liver surgery, such as ICG fluorescence, can be very helpful in the detection of malignant liver lesions, and the resection margins can be determined very precisely in combination with intra-operative ultrasound. The oncological result can be optimized in this way [15,24]. Although laparoscopy has the theoretical advantage of haptics, this limitation in robotic-assisted surgery may be able to be overcome via visual cues [25].
The aim of this systematic review and meta-analysis is to evaluate the influence of robotic liver surgery on the resection margin status in malignant cases compared to the conventional laparoscopic approach.
2. Methods
2.1. Literature Search Strategy
A systematic literature search was performed using PubMed and Cochrane Library. Two authors (M.R. and R.C.) independently conducted the systematic search of the articles in English since 2010. The research ended on 2 July 2021. In the event of disagreement, the case was discussed with the assistance of the third author (A.P.). The search terms were “laparoscopy”, “laparoscop*”,“laparoscopic surgery”, “robotics”, “robot*”, “robotic surgery”, “hepatectomy”, “liver resection”, “liver surgery” and “hepatic resection.” These terms were used with help of the boolean operators AND/OR in different combinations and partly using Medical Subject Headings (MeSH). We also manually searched the reference lists of recent systematic reviews and eligible articles for potentially relevant studies for this work.
2.2. Aim of Study
The primary aim of our meta-analysis was to compare the robotic and laparoscopic procedures with regard to resection margin status after resection of liver malignancies. Secondarily, the perioperative outcomes, such as operation time, intraoperative blood loss, length of hospital stay, tumor size, overall and severe complications and conversion rate, should be analyzed comparatively between robotic and laparoscopic resections of liver lesions, including non-malignant cases.
2.3. Inclusion Criteria
Only studies comparing robotic and laparoscopic liver resections were considered for this meta-analysis. Studies had to include an adequate comparative analysis of laparoscopic and robotic procedures. Above all, the analysis and comparison of the resection margin status in both groups had to be available. Furthermore, the article had to deal with malignant liver lesions, or it had to clearly differentiate between malignant and benign cases with the associated rates of the resection margin status. The malignant cases could include hepatocellular carcinoma, cholangiocarcinoma, colorectal liver metastases and other liver malignancies. Only articles in English were considered.
2.4. Exclusion Criteria
The studies without information on the resection margin status or without clear differentiation between malignant and benign cases were excluded. As mentioned, articles in any other language without an English version were excluded. Furthermore, letters, editorials, study protocols, review articles and meta-analyses without original data, case reports and studies with total numbers of cases <20 were excluded. Studies with overlapping data were excluded, and those that were more suitable for our meta-analysis (i.e., studies with more detailed information on R status, a higher number of malignant cases and higher study quality) were retained. Hand-assisted cases were excluded from the meta-analysis.
2.5. Data Extraction and Quality Assessment
The data were extracted and tabulated, in accordance with inclusion and exclusion criteria: name of first author, year of publication, country where the study was conducted, study design, case number in each of the robotic and laparoscopic groups, number of malignant cases, number of cases with positive resection margins, age and sex of patients, operative time, intra-operative blood loss, length of stay, size of lesion, complications and conversion rate.
The methodological quality of the studies was assessed using the Newcastle–Ottawa scale (NOS) [26]. According to the NOS, points from 0–9 were awarded per study. The studies with scores ≥ 6 were considered to be of high quality.
2.6. Statistical Analysis
This systematic review and meta-analysis were carried out in accordance with the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and the protocol established by the authors, taking into account the inclusion and exclusion criteria [27]. Continuous and dichotomous data were analyzed using mean differences (MD) and odd ratios (OR), respectively. The Mantel–Haenszel method was applied for dichotomous variables. When reporting the continuous data as the median and range or interquartile range, we used the method described by Lou et al. and Wan et al. to calculate the mean and standard deviation [28,29]. p-values of <0.05 were considered to be statistically significant.
The I2 statistic was used to estimate statistical heterogeneity. With I2 ≥ 50% and a significance level of p < 0.05, high heterogeneity was assumed. In this case, we used the random effects model; otherwise, the fixed effects model was used.
We used the RevMan 5.3 software (The Cochrane Collaboration, Oxford, UK) for data analysis.
3. Results
3.1. Results of the Literature Search
A total of 645 records were screened for the inclusion and exclusion criteria. Of these, 50 full-text articles were checked for eligibility. Fourteen of these studies were included in the qualitative and quantitative synthesis (Figure 1). All of them were retrospective in nature. Completed randomized controlled trials were not found. Mejia et al. analyzed and reported the minor and major liver resections separately [30]. Therefore, the data of this study were split accordingly. Table 1 shows characteristics of the included studies. The results of our meta-analysis are summarized in Table 2.
Figure 1.
PRISMA flow diagram of the literature research.
Table 1.
Characteristics of the included studies.
| Author | Year | Country | Study Design | Approach | Cases (n) |
Malignant Cases (n) | Positive RM (n) | Sex (m/f) |
Study Quality (NOS) |
|---|---|---|---|---|---|---|---|---|---|
| Berber [31] | 2010 | USA | RCS | RLR | 9 | 9 | 0 | 7/2 | 9 |
| LLR | 23 | 23 | 0 | 12/11 | |||||
| Troisi [9] | 2013 | Belgium/Italy | RCS | RLR | 40 | 28 | 3 | 27/13 | 7 |
| LLR | 223 | 134 | 12 | 98/125 | |||||
| Spampinato [32] | 2014 | Italy | RCS | RLR | 25 | 17 | 0 | 13/12 | 8 |
| LLR | 25 | 23 | 2 | 10/15 | |||||
| Croner [33] | 2016 | Germany | RCS | RLR | 10 | 10 | 0 | 8/2 | 9 |
| LLR | 19 | 15 | 0 | 13/6 | |||||
| Lai [34] | 2016 | China | RCS | RLR | 100 | 100 | 4 | 66/29 | 7 |
| LLR | 35 | 35 | 3 | 26/9 | |||||
| Lee [10] | 2016 | China | RCS | RLR | 70 | 52 | 1 | 46/24 | 9 |
| LLR | 66 | 57 | 1 | 39/27 | |||||
| Magistri [35] | 2017 | Italy | RCS | RLR | 22 | 22 | 1 | 18/4 | 9 |
| LLR | 24 | 24 | 1 | 15/9 | |||||
| Fruscione [36] | 2019 | USA | RCS | RLR | 57 | 37 | 3 | 20/37 | 7 |
| LLR | 116 | 54 | 4 | 52/64 | |||||
| Hu [37] | 2019 | China | RCS | RLR | 58 | 36 | 0 | 33/25 | 9 |
| LLR | 54 | 26 | 0 | 26/28 | |||||
| Lim [38] | 2019 | France/Italy | RCS | RLR | 61 | 61 | 7 | 41/20 | 8 |
| LLR | 111 | 111 | 17 | 83/28 | |||||
| Marino [39] | 2019 | Italy | RCS | RLR | 14 | 12 | 1 | 8/6 | 8 |
| LLR | 20 | 20 | 3 | 11/9 | |||||
| Mejia (a) [30] | 2020 | USA | RCS | RLR | 35 | 22 | 2 | 16/19 | 8 |
| LLR | 85 | 32 | 3 | 36/49 | |||||
| Mejia (b) [30] | 2020 | USA | RCS | RLR | 8 | 7 | 0 | 4/4 | 8 |
| LLR | 13 | 4 | 1 | 6/7 | |||||
| Cai [40] | 2021 | China | RCS | RLR | 25 | 12 | 0 | 12/13 | 9 |
| LLR | 27 | 15 | 0 | 18/9 | |||||
| Lorenz [41] | 2021 | Germany | RCS | RLR | 44 | 32 | 2 | 24/20 | 8 |
| LLR | 111 | 58 | 7 | 50/61 |
LLR = laparoscopic liver resection, NOS = Newcastle–Ottawa scale, RCS = retrospective cohort study, RLR = robotic liver resection, RM = resection margin.
Table 2.
Summary of the meta-analysis for robotic versus laparoscopic liver resections.
| Outcomes | Studies | Cases (n) | OR/MD | 95% CI | p-Value | Heterogeneity | ||
|---|---|---|---|---|---|---|---|---|
| (n) | RLR/LLR | I2 (%) | p-Value | Model | ||||
| Positive resection margin | 14 | 457/631 | 0.71 | 0.42–1.18 | 0.18 | 0 | 0.98 | FE |
| Operation time | 13 | 565/894 | 28.12 | 3.66–52.57 | 0.02 | 90 | <0.00001 | RE |
| Intra-operative blood loss | 11 | 404/748 | −8.56 | −70.86–53.73 | 0.79 | 82 | <0.00001 | RE |
| Length of stay | 11 | 531/846 | −0.02 | −0.56–0.53 | 0.94 | 76 | <0.00001 | RE |
| Tumor size | 10 | 433/557 | 6.92 | 2.93–10.91 | 0.0007 | 52 | 0.02 | RE |
| Overall complications | 13 | 534/841 | 0.78 | 0.56–1.09 | 0.15 | 21 | 0.23 | FE |
| Severe complications | 8 | 284/492 | 0.92 | 0.51–1.68 | 0.79 | 2 | 0.42 | FE |
| Conversion | 10 | 426/622 | 0.74 | 0.44–1.23 | 0.25 | 44 | 0.07 | FE |
CI = confidence interval, FE = fixed effects model, LLR = laparoscopic liver resection, MD = mean difference, OR = odds ratio, RE = random effects model, RLR = robotic liver resection.
3.2. Resection Margin Status
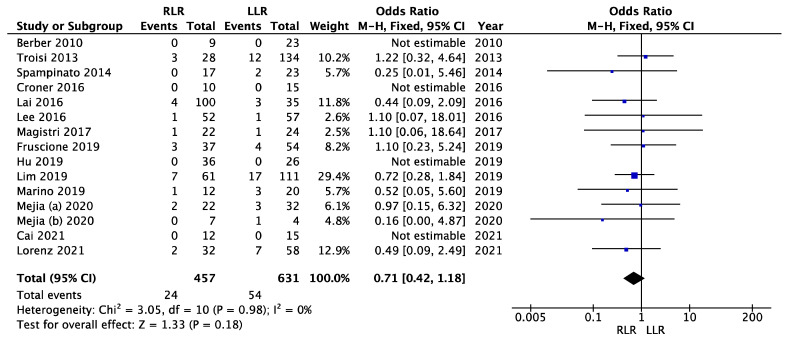
In 14 studies, the resection margin status could be clearly assigned to malignant cases. Our meta-analysis included a total of 1530 cases. Of these, 1088 cases (71.1%) were malignancies: 457 cases in the robotic group vs. 631 cases in the laparoscopic group. Figure 2 shows the forest plot of the meta-analysis on positive resection margin status. There was no significant heterogeneity (I2 = 0%, p = 0.98), so we used the fixed effects model. No significant difference could be shown in the meta-analysis of the positive resection margin status between the robotic and laparoscopic approaches (OR = 0.71; 95% CI (0.42–1.18); p = 0.18).
Figure 2.
Meta-analysis of positive resection margin status.
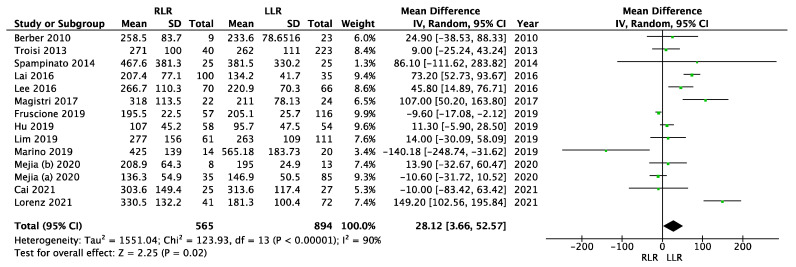
3.3. Operation Time
There was high heterogeneity (I2 = 90%, p < 0.00001), so we used the random effects model for meta-analysis of operative time (Figure 3). The operative time was significantly higher in the robotic group (MD = 28.12; 95% CI (3.66–52.57); p = 0.02).
Figure 3.
Meta-analysis of operation time.
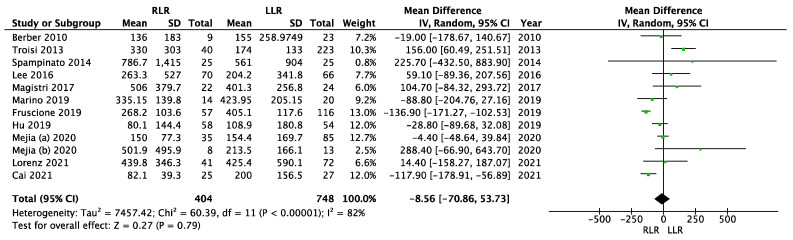
3.4. Intra-Operative Blood Loss
Thirteen studies reported the intra-operative blood loss, one of them without standard deviation or ranges and another one with mean and range, so these studies were not considered for the meta-analysis of the intra-operative blood loss [33,34]. Lim et al. did not report on intra-operative blood loss [38]. High heterogeneity was observed (I2 = 82%, p < 0.00001). The random effects model was used (Figure 4). There was no significant difference in intra-operative blood loss between the groups (MD = −8.56; 95% CI (−70.86–53.73); p = 0.79).
Figure 4.
Meta-analysis of intra-operative blood loss.
3.5. Length of Hospital Stay
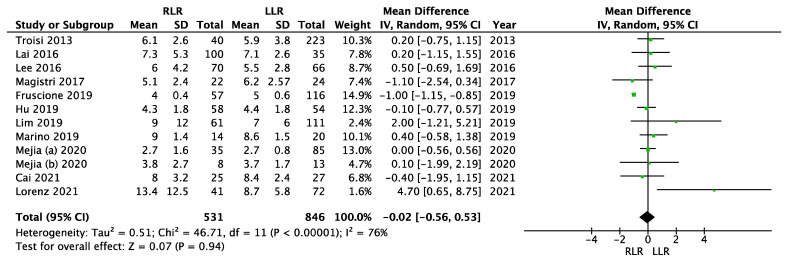
The meta-analysis showed no significant difference between the robotic and laparoscopic groups regarding the length of hospital stay (MD = −0.02; 95% CI (−0.56–0.53); p = 0.94). We used a random effects model. There was significant heterogeneity (I2 = 76%, p < 0.00001). Figure 5 shows the meta-analysis of length of hospital stay.
Figure 5.
Meta-analysis of length of hospital stay.
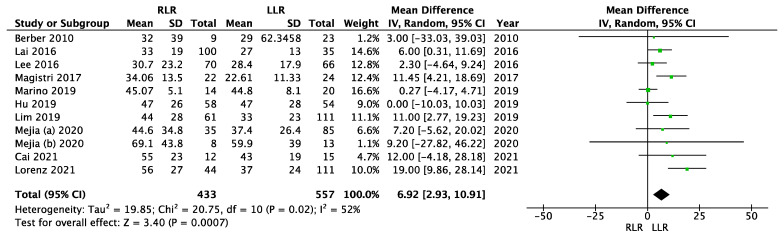
3.6. Tumor Size
Data from ten studies were used for the meta-analysis of tumor size (Figure 6). Two studies did not report the data on tumor size [32,36]. One study presented data as mean and range [33]. In one study, data on tumor size were inconclusive [9]. Therefore, these four studies were excluded from the meta-analysis of tumor size. There was significant heterogeneity (I2 = 52%, p = 0.02), so we used a random effects model. The meta-analysis showed that the tumor size was significantly larger in the robotic group (MD = 6.92; 95% CI (2.93–10.91); p = 0.0007).
Figure 6.
Meta-analysis of tumor size.
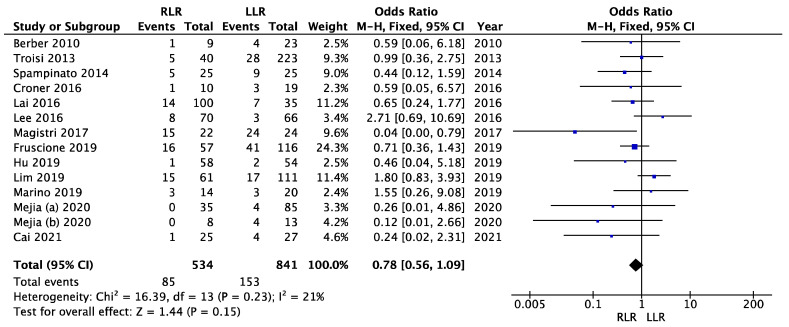
3.7. Overall Complications
All studies reported data on complications. One study reported only the severe complications (Clavien-Dindo grade ≥ 3), so it was excluded from the meta-analysis of overall complications [41]. No significant heterogeneity was observed (I2 = 21%, p = 0.23). A fixed effects model was used. There was no significant difference between the groups with regard to overall complications (OR = 0.78; 95% CI (0.56–1.09); p = 0.15). Figure 7 illustrates the meta-analysis of overall complications.
Figure 7.
Meta-analysis of overall complications.
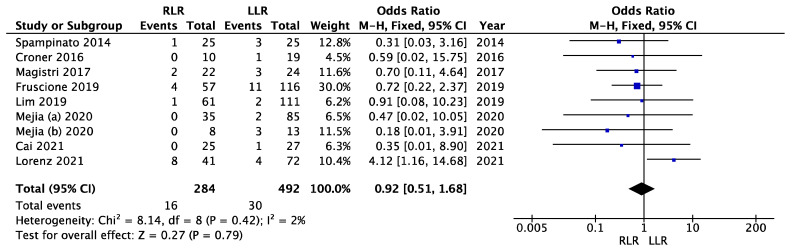
3.8. Severe Complications
In eight studies, severe complications (Clavien-Dindo grade ≥ 3) were reported or could be clearly differentiated (Figure 8). There was no significant heterogeneity (I2 = 2%, p = 0.42). We used the fixed effects model. The meta-analysis showed no significant difference between the robotic and laparoscopic approaches regarding severe complications (OR = 0.92; 95% CI (0.51–1.68); p = 0.79).
Figure 8.
Meta-analysis of severe complications.
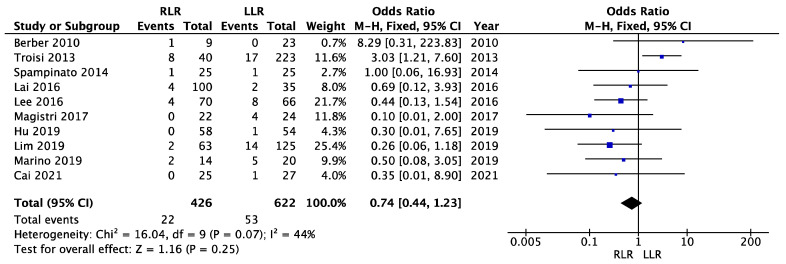
3.9. Conversion
There was not high heterogeneity regarding the conversion rate (I2 = 44%, p = 0.07). The fixed effects model was used (Figure 9). There was no significant difference between the groups in terms of conversion rate (OR = 0.74; 95% CI (0.44–1.23); p = 0.25).
Figure 9.
Meta-analysis of conversion.
3.10. Liver Malignancies
In our study, 1088 malignant cases were identified. These included 604 hepatocellular carcinomas and 64 cholangiocarcinomas (Table 3). Colorectal liver metastases were detected in at least 305 cases. Fruscione et al. reported liver metastases in 24 cases in the robotic group and 31 cases in the laparoscopic group in their study [36]. It was unclear whether or how many of these were colorectal liver metastases. Cai et al. reported one metastasis in the laparoscopic group in their study [40]. It was also unclear whether it was a colorectal liver metastasis. The remaining malignant cases were other liver malignancies.
Table 3.
Liver malignancies.
| Author | Year | Approach | HCC | CCA | CRLM | Other Malignancies |
|---|---|---|---|---|---|---|
| Berber [31] | 2010 | RLR | 3 | 1 | 4 | 1 |
| LLR | 7 | 0 | 14 | 2 | ||
| Troisi [9] | 2013 | RLR | 3 | 1 | 24 | 0 |
| LLR | 9 | 2 | 108 | 15 | ||
| Spampinato [32] | 2014 | RLR | 2 | 2 | 11 | 2 |
| LLR | 1 | 3 | 16 | 3 | ||
| Croner [33] | 2016 | RLR | 4 | 1 | 5 | 0 |
| LLR | 5 | 2 | 5 | 3 | ||
| Lai [34] | 2016 | RLR | 100 | 0 | 0 | 0 |
| LLR | 35 | 0 | 0 | 0 | ||
| Lee [10] | 2016 | RLR | 40 | 3 | 8 | 1 |
| LLR | 41 | 1 | 13 | 2 | ||
| Magistri [35] | 2017 | RLR | 22 | 0 | 0 | 0 |
| LLR | 24 | 0 | 0 | 0 | ||
| Fruscione [36] | 2019 | RLR | 4 | 7 | uc | uc |
| LLR | 16 | 7 | uc | uc | ||
| Hu [37] | 2019 | RLR | 25 | 4 | 2 | 5 |
| LLR | 23 | 1 | 2 | 0 | ||
| Lim [38] | 2019 | RLR | 42 | 2 | 15 | 2 |
| LLR | 72 | 6 | 23 | 10 | ||
| Marino [39] | 2019 | RLR | 4 | 0 | 8 | 0 |
| LLR | 7 | 0 | 13 | 0 | ||
| Mejia (a) [30] | 2020 | RLR | 18 | 1 | 2 | 1 |
| LLR | 26 | 0 | 6 | 0 | ||
| Mejia (b) [30] | 2020 | RLR | 4 | 2 | 1 | 0 |
| LLR | 4 | 0 | 1 | 0 | ||
| Cai [40] | 2021 | RLR | 8 | 3 | 0 | 1 |
| LLR | 9 | 5 | uc | uc | ||
| Lorenz [41] | 2021 | RLR | 13 | 5 | 12 | 2 |
| LLR | 33 | 4 | 12 | 9 |
CCA = cholangiocarcinoma, CRLM = colorectal liver metastasis, HCC = hepatocellular carcinoma, LLR = laparoscopic liver resection, RLR = robotic liver resection, uc = unclear.
4. Discussion
Robotic procedures have become indispensable in modern liver surgery. The safety and feasibility of this approach is no longer a topic of discussion. However, the advantages of the robotic approach in comparison to conventional laparoscopic and open procedures in liver surgery are the subject of considerable debate [1]. Except for the longer operative time and higher costs of robotic liver surgery, robotic and laparoscopic approaches to liver surgery have largely similar peri-operative results. There were no significant differences between the two procedures with regard to blood loss, blood transfusion, length of hospital stay, tumor-free resection margin or complication rate in the previous analyses [2,3,4].
In our study, the operation time was significantly longer in the robotic group than in the laparoscopic group (MD = 28.12; 95% CI (3.66–52.57); p = 0.02). There were no significant differences between procedures in terms of intra-operative blood loss, length of hospital stay, overall and severe complications or conversion rate.
Our meta-analysis included a total of 1530 cases. Malignancies were identified in 71.1% (n = 1088) of these cases. A positive resection margin was observed in 5.3% of cases (n = 24) in the robotic group and in 8.6% of cases (n = 54) in the laparoscopic group. However, this difference was not statistically significant (OR = 0.71; 95% CI (0.42–1.18); p = 0.18). Nevertheless, there was a trend in favor of robotic liver surgery, considering previous analyses. Montalti et al. compared 155 vs. 395 liver resections in robotic and laparoscopic groups, respectively, for resection margin status in their meta-analysis. There were 23 cases (14.8%) in the robotic group and 33 cases (8.4%) in the laparoscopic group with positive resection margins (OR = 1.71; 95% CI (0.95–3.09); p = 0.07) [12]. The meta-analysis by Guan et al. included nine studies with 345 cases in the robotic group and 396 cases in the laparoscopic group for analysis of R status. Positive resection margins were noted in 27 cases (7.8%) in the robotic group and in 33 cases (8.3%) in the laparoscopic group (OR = 1.03; 95% CI (0.41–2.55); p = 0.95) [13]. In the pooled analysis of minor liver resections by Wang et al., R0 resection was achieved in 167 (96.0%) of 174 robotic resections and 181 (95.3%) of 190 laparoscopic resections (OR = 1.36; 95% CI (0.48 to 3.83); p = 0.56) [4]. However, it should be noted that some studies did not differentiate between malignant and benign cases when reporting the rates of positive resection margins, so the percentages were reported from the entire cohort [9,14]. These numbers were used in some meta-analyses without further differentiation [11,12,13]. In many studies, the number of cases in the laparoscopic liver surgery group is higher than in the robotic liver surgery group due to the earlier adoption of the laparoscopic approach. If no attention is paid to the precise differentiation of malignant and benign cases when interpreting the R0 or R1 rates, this can lead to a lower percentage of positive resection margins.
Furthermore, our meta-analysis showed that significantly larger liver lesions were resected with the robot procedure compared to the laparoscopic procedure (MD = 6.92; 95% CI (2.93–10.91); p = 0.0007). This finding was consistent with the results of previous meta-analyses [3,42]. Hu et al. were able to demonstrate significantly larger tumor size in the robotic group based on the data from five studies [3]. Zhang et al. compared the tumor sizes of 743 cases in the robotic group and 1,132 cases in the laparoscopic group in their meta-analysis. In this study, tumor size was significantly larger in the robotic group (WMD = 0.36; 95% CI (0.16–0.56); p < 0.001) [42].
Higher freedom of movement, stable three-dimensional visualization, the possibility of using a third arm and the absence of a physiological tremor are the advantages of robotic over conventional laparoscopic surgery. These advantages of robotics enable us to operate safely and precisely in the tight areas and difficult-to-access localizations of the liver [21,22,23]. One of the modern approaches in robot-assisted liver resection is image-guided surgery. Intraoperative navigation can be facilitated using augmented reality during robotic liver surgery. Based on the information from the preoperative and/or intraoperative imaging, 3D reconstructions of the liver can be created in which tumor, intrahepatic bile and vascular structures can be visualized and marked in color. In this way, the operator can better orientate himself/herself during the parenchyma dissection using these virtual landmarks [43]. In addition to the safety distance, a constant dissection of the parenchyma and not leaving the previously defined resection plane are important factors in achieving an R0 resection. Due to the advantages of robotics mentioned above, these properties could be better ensured by the robot. All of these factors may also have contributed to surgeons daring to operate on larger lesions robotically than laparoscopically.
Perhaps the most discussed limitation of the robot-assisted approach is the lack of haptics when compared to standard laparoscopy. As mentioned above, many robotics surgeons believe that the above-mentioned advantages, combined with visual cues, render this theoretical deficiency moot. This review is limited by the fact that tumor location was not taken into account. Future studies need to report tumor location in the posterior or anterior segments and proximity to major hepatic blood vessels to more accurately compare these two approaches. Another confounding factor is the possibility that many robotic surgeons have long experience with laparoscopic liver surgery prior to embarking on robotic-assisted liver resections. The relevance of a minimally invasive surgeon’s previous surgical experience has been highlighted by a recent publication that discusses the initiation, standardization and proficiency phases of the learning curve according to where along the IDEAL (Idea, Development, Exploration, Assessment and Long-term) framework surgeons fall [44].
5. Conclusions
With regard to the resection margin status, no significant difference between the robotic and laparoscopic procedures could be determined in the pooled analysis. Tumor size was significantly larger in the robotic group. However, due to the limitations of the published data, randomized controlled trials are needed to truly delineate any potential benefits of robotics in liver resection.
Acknowledgments
Not applicable.
Author Contributions
Conceptualization: M.R., A.P., M.A.H., A.A.G. and R.S.C. Data extraction: M.R., A.P. and R.S.C. Data curation: M.R., A.P., M.A., J.S., M.F., J.A. and R.S.C. Investigation: M.R., A.P. and R.S.C. Methodology: M.R., A.P., S.A.-M., M.A.H. and R.S.C. Writing—original draft: M.R. and R.S.C. Writing—review and editing: M.R., A.P., M.A., J.S., M.F., J.A., S.A.-M., A.A.G. and R.S.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are provided in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu R., Wakabayashi G., Kim H.-J., Choi G.-H., Yiengpruksawan A., Fong Y., He J., Boggi U., I Troisi R., Efanov M., et al. International consensus statement on robotic hepatectomy surgery in 2018. World J. Gastroenterol. 2019;25:1432–1444. doi: 10.3748/wjg.v25.i12.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qiu J., Chen S., Chengyou D. A systematic review of robotic-assisted liver resection and meta-analysis of robotic versus laparoscopic hepatectomy for hepatic neoplasms. Surg. Endosc. 2016;30:862–875. doi: 10.1007/s00464-015-4306-7. [DOI] [PubMed] [Google Scholar]
- 3.Hu Y., Guo K., Xu J., Xia T., Wang T., Liu N., Fu Y. Robotic versus laparoscopic hepatectomy for malignancy: A systematic review and meta-analysis. Asian J. Surg. 2021;44:615–628. doi: 10.1016/j.asjsur.2020.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Wang J.M., Li J.F., Yuan G.D., He S.Q. Robot-assisted versus laparoscopic minor hepatectomy: A systematic review and meta-analysis. Medicine (Baltimore) 2021;100:e25648. doi: 10.1097/MD.0000000000025648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agrawal S., Belghiti J. Oncologic resection for malignant tumors of the liver. Ann. Surg. 2011;253:656–665. doi: 10.1097/SLA.0b013e3181fc08ca. [DOI] [PubMed] [Google Scholar]
- 6.Centonze L., De Carlis R., Vella I., Carbonaro L., Incarbone N., Palmieri L., Sgrazzutti C., Ficarelli A., Valsecchi M.G., Iacono U.D., et al. From LI-RADS Classification to HCC Pathology: A Retrospective Single-Institution Analysis of Clinico-Pathological Features Affecting Oncological Outcomes after Curative Surgery. Diagnostics. 2022;12:160. doi: 10.3390/diagnostics12010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spolverato G., Yakoob M.Y., Kim Y., Alexandrescu S., Marques H.P., Lamelas J., Aldrighetti L., Gamblin T.C., Maithel S.K., Pulitano C., et al. The Impact of Surgical Margin Status on Long-Term Outcome After Resection for Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2015;22:4020–4028. doi: 10.1245/s10434-015-4472-9. [DOI] [PubMed] [Google Scholar]
- 8.Eveno C., Karoui M., Gayat E., Luciani A., Auriault M., Kluger M.D., Baumgaertner I., Baranes L., Laurent A., Tayar C., et al. Liver resection for colorectal liver metastases with peri-operative chemotherapy: Oncological results of R1 resections. HPB (Oxford) 2013;15:359–364. doi: 10.1111/j.1477-2574.2012.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Troisi R.I., Patriti A., Montalti R., Casciola L. Robot assistance in liver surgery: A real advantage over a fully laparoscopic approach? Results of a comparative bi-institutional analysis. Int. J. Med. Robot. 2013;9:160–166. doi: 10.1002/rcs.1495. [DOI] [PubMed] [Google Scholar]
- 10.Lee K.-F., Cheung Y.-S., Chong C.C.N., Wong J., Fong A.K.W., Lai P.B.S. Laparoscopic and robotic hepatectomy: Experience from a single centre. ANZ J. Surg. 2016;86:122–126. doi: 10.1111/ans.13339. [DOI] [PubMed] [Google Scholar]
- 11.Kamarajah S.K., Bundred J., Manas D., Jiao L.R., Abu Hilal M., White S.A. Robotic versus conventional laparoscopic liver resections: A systematic review and meta-analysis. Scand. J. Surg. 2021;110:290–300. doi: 10.1177/1457496920925637. [DOI] [PubMed] [Google Scholar]
- 12.Montalti R., Berardi G., Patriti A., Vivarelli M., Troisi R.I. Outcomes of robotic vs laparoscopic hepatectomy: A systematic review and meta-analysis. World J. Gastroenterol. 2015;21:8441–8451. doi: 10.3748/wjg.v21.i27.8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan R., Chen Y., Yang K., Ma D., Gong X., Shen B., Peng C. Clinical efficacy of robot-assisted versus laparoscopic liver resection: A meta analysis. Asian J. Surg. 2019;42:19–31. doi: 10.1016/j.asjsur.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Montalti R., Scuderi V., Patriti A., Vivarelli M., Troisi R.I. Robotic versus laparoscopic resections of posterosuperior segments of the liver: A propensity score-matched comparison. Surg. Endosc. 2016;30:1004–1013. doi: 10.1007/s00464-015-4284-9. [DOI] [PubMed] [Google Scholar]
- 15.Marino M.V., Podda M., Fernandez C.C., Ruiz M.G., Fleitas M.G. The application of indocyanine green-fluorescence imaging during robotic-assisted liver resection for malignant tumors: A single-arm feasibility cohort study. HPB (Oxford) 2020;22:422–431. doi: 10.1016/j.hpb.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Rahimli M., Perrakis A., Schellerer V., Gumbs A., Lorenz E., Franz M., Arend J., Negrini V.-R., Croner R.S. Robotic and laparoscopic liver surgery for colorectal liver metastases: An experience from a German Academic Center. World J. Surg. Oncol. 2020;18:333. doi: 10.1186/s12957-020-02113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Z., Yin Z., Pan L., Li C., Hu M., Lau W.Y., Liu R. Robotic hepatic resection in postero-superior region of liver. Updates Surg. 2021;73:1007–1014. doi: 10.1007/s13304-020-00895-3. [DOI] [PubMed] [Google Scholar]
- 18.Morelli L., Guadagni S., Furbetta N., Di Franco G., Palmeri M., Gianardi D., Bianchini M., Guadagnucci M., Pollina L., Masi G., et al. Robotic-assisted surgery for colorectal liver metastasis: A single-centre experience. J. Minim. Access Surg. 2019;16:160–165. doi: 10.4103/jmas.JMAS_265_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Croner R.S., Perrakis A., Brunner M., Matzel K.E., Hohenberger W. Pioneering Robotic Liver Surgery in Germany: First Experiences with Liver Malignancies. Front. Surg. 2015;2:18. doi: 10.3389/fsurg.2015.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerra F., Bonapasta S.A., Annecchiarico M., Bongiolatti S., Coratti A. Robot-integrated intraoperative ultrasound: Initial experience with hepatic malignancies. Minim. Invasive Ther. Allied Technol. 2015;24:345–349. doi: 10.3109/13645706.2015.1022558. [DOI] [PubMed] [Google Scholar]
- 21.Di Sandro S., Danieli M., Ferla F., Lauterio A., De Carlis R., Benuzzi L., Buscemi V., Pezzoli I., De Carlis L. The current role of laparoscopic resection for HCC: A systematic review of past ten years. Transl. Gastroenterol. Hepatol. 2018;3:68. doi: 10.21037/tgh.2018.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perrakis A., Rahimli M., Gumbs A.A., Negrini V., Andric M., Stockheim J., Wex C., Lorenz E., Arend J., Franz M., et al. Three-Device (3D) Technique for Liver Parenchyma Dissection in Robotic Liver Surgery. J. Clin. Med. 2021;10:5265. doi: 10.3390/jcm10225265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Croner R.S., Gumbs A.A., Perrakis A., Andric M., Stockheim J., Lorenz E., Arend J., Franz M., Rahimli M. Robotic vs. laparoscopic liver surgery: What are the advantages of the robot? Dig. Med. Res. 2021;4:1–10. doi: 10.21037/dmr-21-26. [DOI] [Google Scholar]
- 24.Franz M., Arend J., Wolff S., Perrakis A., Rahimli M., Negrini V.-R., Stockheim J., Lorenz E., Croner R. Tumor visualization and fluorescence angiography with indocyanine green (ICG) in laparoscopic and robotic hepatobiliary surgery-valuation of early adopters from Germany. Innov. Surg. Sci. 2021;6:59–66. doi: 10.1515/iss-2020-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gumbs A.A., Abu-Hilal M., Tsai T.-J., Starker L., Chouillard E., Croner R. Keeping surgeons in the loop: Are handheld robotics the best path towards more autonomous actions? (A comparison of complete vs. handheld robotic hepatectomy for colorectal liver metastases) Artif. Intell. Surg. 2021;1:38–51. doi: 10.20517/ais.2021.07. [DOI] [Google Scholar]
- 26.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 27.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo D., Wan X., Liu J., Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018;27:1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 29.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mejia A., Cheng S.S., Vivian E., Shah J., Oduor H., Archarya P. Minimally invasive liver resection in the era of robotics: Analysis of 214 cases. Surg. Endosc. 2019;34:339–348. doi: 10.1007/s00464-019-06773-3. [DOI] [PubMed] [Google Scholar]
- 31.Berber E., Akyildiz H.Y., Aucejo F., Gunasekaran G., Chalikonda S., Fung J. Robotic versus laparoscopic resection of liver tumours. HPB (Oxford) 2010;12:583–586. doi: 10.1111/j.1477-2574.2010.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spampinato M.G., Coratti A., Bianco L., Caniglia F., Laurenzi A., Puleo F., Ettorre G.M., Boggi U. Perioperative outcomes of laparoscopic and robot-assisted major hepatectomies: An Italian multi-institutional comparative study. Surg. Endosc. 2014;28:2973–2979. doi: 10.1007/s00464-014-3560-4. [DOI] [PubMed] [Google Scholar]
- 33.Croner R.S., Perrakis A., Hohenberger W., Brunner M. Robotic liver surgery for minor hepatic resections: A comparison with laparoscopic and open standard procedures. Langenbeck’s Arch. Surg. 2016;401:707–714. doi: 10.1007/s00423-016-1440-1. [DOI] [PubMed] [Google Scholar]
- 34.Lai E.C., Tang C.N. Long-term Survival Analysis of Robotic Versus Conventional Laparoscopic Hepatectomy for Hepatocellular Carcinoma: A Comparative Study. Surg. Laparosc. Endosc. Percutan Tech. 2016;26:162–166. doi: 10.1097/SLE.0000000000000254. [DOI] [PubMed] [Google Scholar]
- 35.Magistri P., Tarantino G., Guidetti C., Assirati G., Olivieri T., Ballarin R., Coratti A., Di Benedetto F. Laparoscopic versus robotic surgery for hepatocellular carcinoma: The first 46 consecutive cases. J. Surg. Res. 2017;217:92–99. doi: 10.1016/j.jss.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Fruscione M., Pickens R., Baker E.H., Cochran A., Khan A., Ocuin L., Iannitti D.A., Vrochides D., Martinie J.B. Robotic-assisted versus laparoscopic major liver resection: Analysis of outcomes from a single center. HPB (Oxford) 2019;21:906–911. doi: 10.1016/j.hpb.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 37.Hu M., Liu Y., Li C., Wang G., Yin Z., Lau W.Y., Liu R. Robotic versus laparoscopic liver resection in complex cases of left lateral sectionectomy. Int. J. Surg. 2019;67:54–60. doi: 10.1016/j.ijsu.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Lim C., Salloum C., Tudisco A., Ricci C., Osseis M., Napoli N., Lahat E., Boggi U., Azoulay D. Short- and Long-term Outcomes after Robotic and Laparoscopic Liver Resection for Malignancies: A Propensity Score-Matched Study. World J. Surg. 2019;43:1594–1603. doi: 10.1007/s00268-019-04927-x. [DOI] [PubMed] [Google Scholar]
- 39.Marino M.V., Shabat G., Guarrasi D., Gulotta G., Komorowski A. Comparative Study of the Initial Experience in Performing Robotic and Laparoscopic Right Hepatectomy with Technical Description of the Robotic Technique. Dig. Surg. 2018;36:241–250. doi: 10.1159/000487686. [DOI] [PubMed] [Google Scholar]
- 40.Cai J.-P., Chen W., Chen L.-H., Wan X.-Y., Lai J.-M., Yin X.-Y. Comparison between robotic-assisted and laparoscopic left hemi-hepatectomy. Asian J. Surg. 2022;45:265–268. doi: 10.1016/j.asjsur.2021.05.017. [DOI] [PubMed] [Google Scholar]
- 41.Lorenz E., Arend J., Franz M., Rahimli M., Perrakis A., Negrini V., Gumbs A.A., Croner R.S. Robotic and laparoscopic liver resection-comparative experiences at a high-volume German academic center. Langenbecks Arch. Surg. 2021;406:753–761. doi: 10.1007/s00423-021-02152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L., Yuan Q., Xu Y., Wang W. Comparative clinical outcomes of robot-assisted liver resection versus laparoscopic liver resection: A meta-analysis. PLoS ONE. 2020;15:e0240593. doi: 10.1371/journal.pone.0240593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giannone F., Felli E., Cherkaoui Z., Mascagni P., Pessaux P. Augmented Reality and Image-Guided Robotic Liver Surgery. Cancers. 2021;13:6268. doi: 10.3390/cancers13246268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gumbs A.A., Abu Hilal M., Croner R., Gayet B., Chouillard E., Gagner M. The initiation, standardization and proficiency (ISP) phases of the learning curve for minimally invasive liver resection: Comparison of a fellowship-trained surgeon with the pioneers and early adopters. Surg. Endosc. 2021;35:5268–5278. doi: 10.1007/s00464-020-08122-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are provided in the manuscript.