Figure 3.
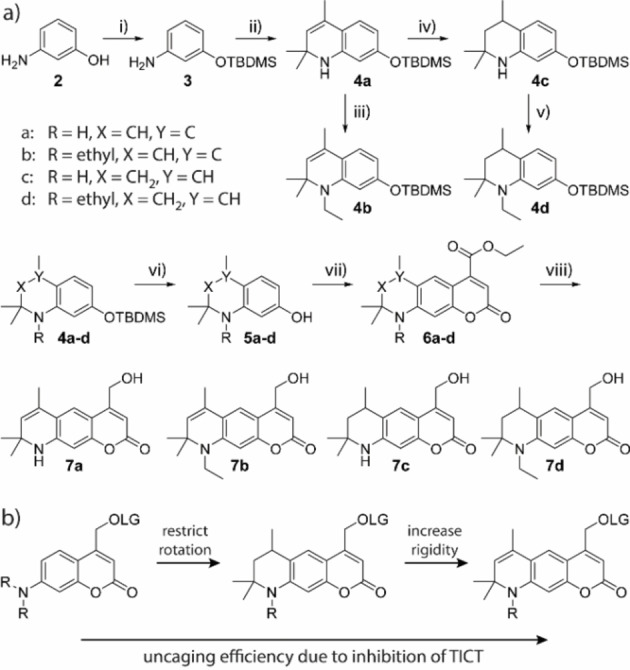
a) Synthesis of compounds 7 a–d. i) TBDMS−Cl, imidazole, DCM, quant. ii) Yb(OTf)3, acetone, 77 %. iii) iodoethane, Cs2CO3, MeCN, 78 %. iv) Pd/C, H2, MeOH, 98 %. v) iodoethane, Cs2CO3, MeCN, 83 %. vi) TBAF, AcOH, THF, 89–94 %. vii) sodium diethyl oxalacetate, EtOH, 31–47 %,. viii) NaBH4, MeOH, 37–62 %. TBDMS=tert‐butyldimethylsilyl. b) Evolution of new coumarin‐photocages with improved uncaging efficiency due to inhibition if intramolecular twisting. LG: leaving group, R: H/ethyl, TICT: twisted intramolecular charge transfer.
