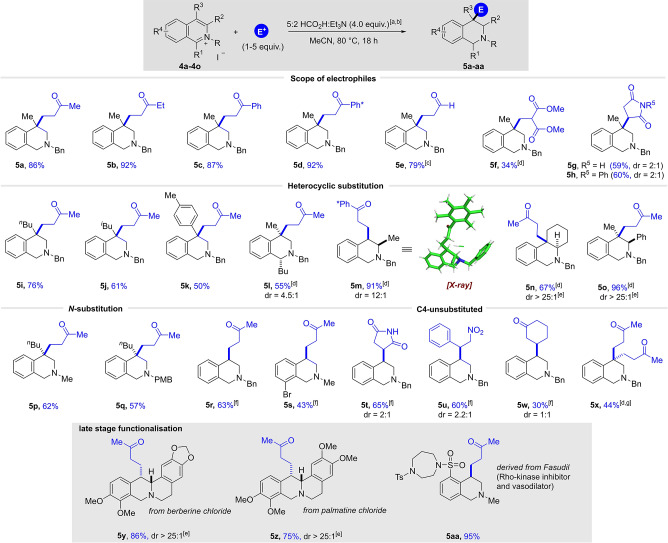
Scheme 3.
Substrate scope for the synthesis of functionalized tetrahydroisoquinolines: [a] Reaction conditions: isoquinolinium salt 4 (1.0 equiv), electrophile (2.0 equiv), 5 : 2 HCO2H : Et3N (4.0 equiv), MeCN (1.25 M), 80 °C, 18 h. [b] Yields refer to isolated material after column chromatography. [c] Reaction conducted in the presence of 0.01 mol % [RhCp*Cl2]2 at 50 °C. [d] Reaction conducted in the presence of 0.01 mol % [RhCp*Cl2]2 at 75 °C. [e] For compound with dr>25 : 1 only one diastereomer was detectable in the crude 1H NMR spectrum. [f] Reaction conducted with 1.0 equiv of electrophile. [g] Reaction conducted with 5.0 equiv of electrophile. The relative stereochemistry of 5 g, 5 h, and 5 t–w is unassigned. The relative stereochemistry of compounds 5 l–o were assigned by NOESY experiments and 5 y–z by J‐coupling (see Supporting Information).