Figure 2.
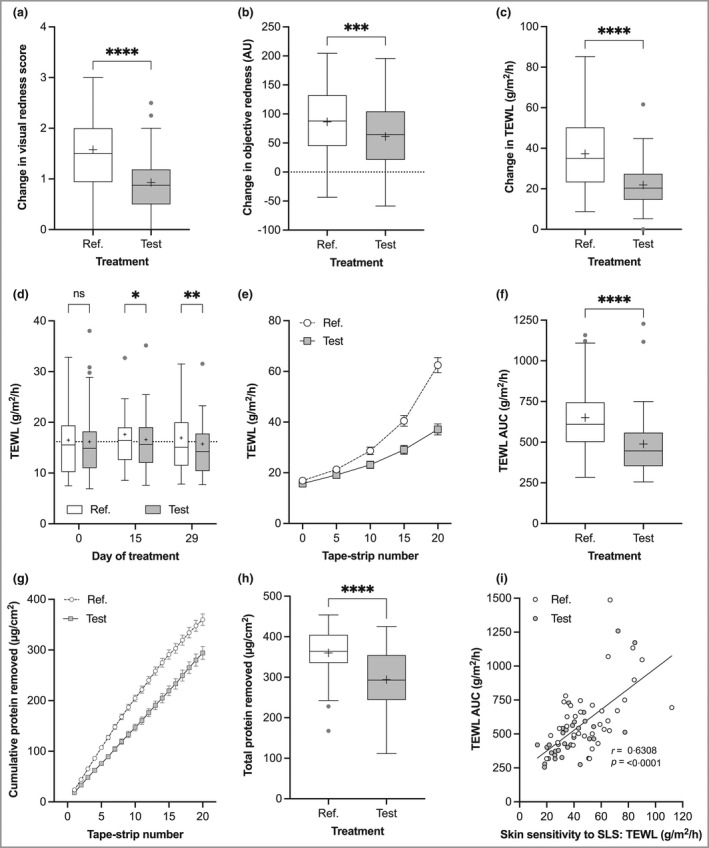
Skin barrier (SB) primary outcomes assessed at forearm sites. (a–c) Skin sensitivity to sodium lauryl sulfate (SLS) following 28 days of treatment measured as the change (day 31, 24 h after patch removal, minus day 29, before patch application): (a) visual redness/erythema, (b) objective redness (arbitrary units, AU) and (c) transepidermal water loss (TEWL). (d) SB function measured as (resting) TEWL before, during (day 15) and after treatment (day 29). (e) TEWL in response to skin tape stripping (STS) (standardized physical skin challenge). (f) SB integrity determined as the area under the TEWL curve (AUC) in response to STS: higher TEWL AUC indicates weaker SB integrity. (g) The cumulative amount of protein removed by STS. (h) The total amount of protein removed by STS. (i) The relationship between TEWL AUC and skin sensitivity to SLS. Boxes indicate the median and 25th and 75th percentiles, with ‘+’ for the mean and whiskers showing 1·5 × interquartile range. Asterisks indicate the results of pairwise testing: ns, not significant; *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001.
