FIGURE 1.
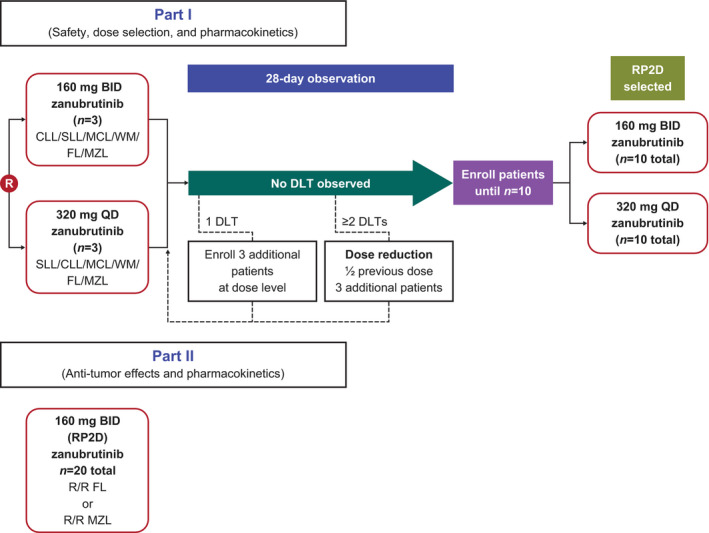
Study design. In part I, three patients (or six patients if one of the first three patients experienced a DLT) were randomly assigned to one of the two treatment arms. If two or more patients experienced a DLT, the dosage was to be reduced by half and three patients were then enrolled at that dose. If zero or one patient experienced a DLT, 10 patients in total were to be enrolled in the arm to further assess the safety, tolerability, PK, and preliminary PD of the study drug and to determine the RP2D. DLT, dose‐limiting toxicity; RP2D, recommended phase II dose
