Figure 7. The WHBP enables recordings of tSNA, showing enlarged inspiratory Resp‐SNA in models of hypertension, likely generated via inspiratory modulation of C1 neurons by pre‐BötC neurons.
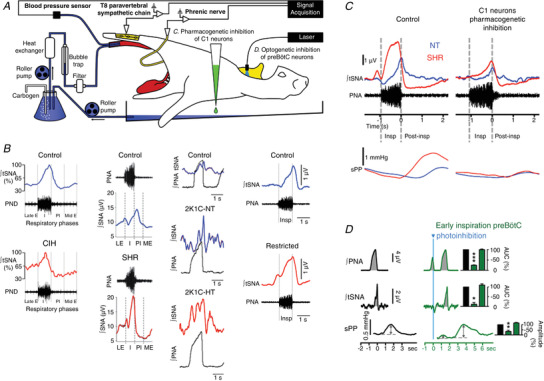
A, recordings of thoracic (T8–T10) vasomotor sympathetic nerve activity (tSNA) in the WHBP, at the level of the paravertebral sympathetic chain. B, when compared to phrenic nerve activity (PNA), tSNA shows phasic respiratory‐locked activity (Resp‐SNA), principally during inspiration, with a peak at the inspiration–post‐inspiration phase transition, as seen in all recordings of control animals (blue). In chronic intermittent hypoxia (CIH) Resp‐SNA (%) is amplified during late expiration. However, in spontaneously hypertensive rat (SHR), 2 kidney 1 clip (2K1C) and uteroplacental insufficiency (restricted) rat models of hypertension, Resp‐SNA is amplified during inspiration with a peak shift into inspiration (red). In most models, the exaggerated Resp‐SNA appears before the development of hypertension. This is highlighted on the 2K1C‐Normotensive (NT) trace, which shows exaggerated Resp‐SNA during inspiration compared to control, even though the animal is normotensive at this stage. Further increases occur at the hypertensive stage (2K1C‐HT). C, pharmacogenetic inhibition of C1 neurons in the WHBP, as shown in A by adding the ligand to the perfusate, decreased Resp‐SNA of SHR (red) to normotensive (NT; blue) level, with a Resp‐SNA peak that is shifted back to the inspiration–post‐inspiration phase transition and decreased Traube–Hering waves in the SHR to NT level, as seen on systolic perfusion pressure (sPP). D, single‐pulse optogenetic inhibition of pre‐BötC neurons during early inspiration in the WHBP, as shown in A by placing optical fibres linked to a laser bilaterally in the medulla oblongata, stopped the on‐going inspiratory burst, strongly decreasing the associated Resp‐SNA and Traube–Hering wave. Adapted from data in Menuet et al. (2017, 2016, 2020), Oliveira‐Sales et al. (2016), Simms et al. (2009), Zoccal et al. (2008).
