Figure 2.
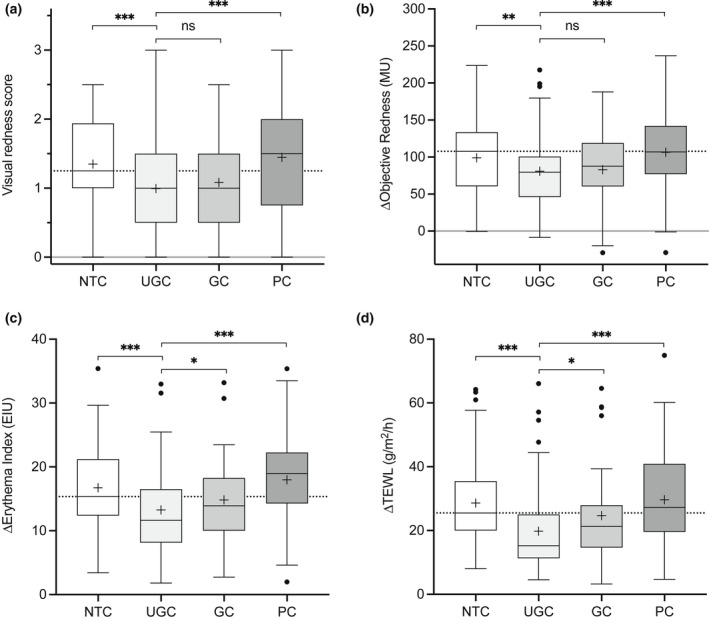
(a–d) Primary outcome of skin sensitivity after 28 days of treatment: (a) visual redness/erythema (Day 31); (b) change in objective redness measured with a Mexameter [in Mexameter units (MU), Day 31 minus Day 29]; (c) change in redness determined from dermoscopic images [Erythema Index units (EIU), Day 31 minus Day 29]; and (d) change in transepidermal water loss (TEWL) (Day 31 minus Day 29). Boxes indicate the median, 25th and 75th percentiles, with ‘+’ for the mean and whiskers showing 1.5 × interquartile range (IQR). Asterisks indicate the results of pairwise testing against UGC (test) (*P < 0.05, **P < 0.01, ***P < 0.001). GC, glycerol cream; NTC, no‐treatment control; PC, paraffin cream; UGC, urea–glycerol cream.
