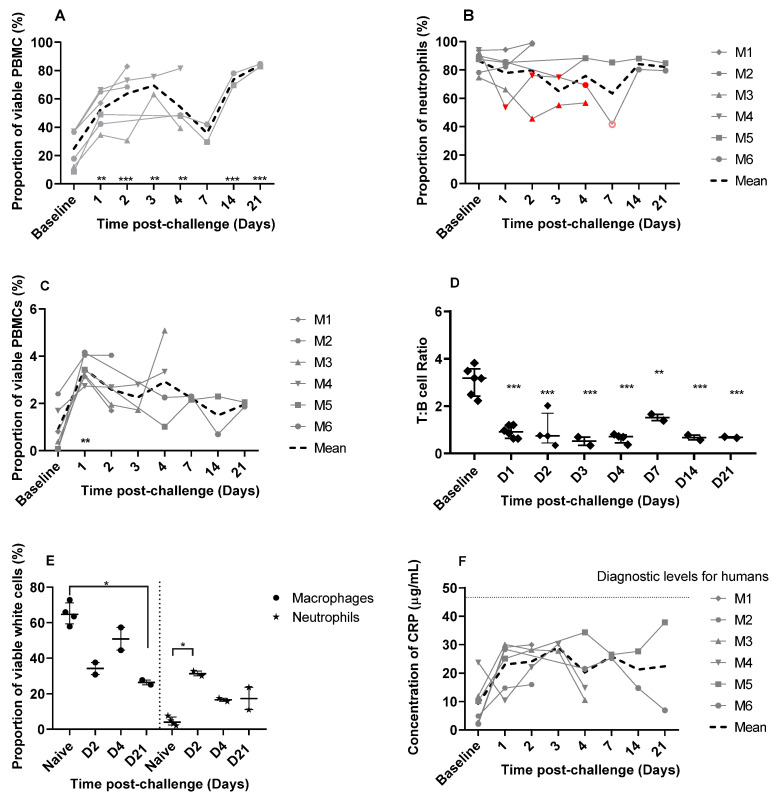
Figure 4.
The immunological response in marmosets following aerosol challenge with SARS-CoV-2 VIC01. (A) The proportion of neutrophils in PBMCs from blood collected prior to, and post-challenge defined by size, granularity, and expression of both CD11c and CD14. (B) HLA-DR expression in circulating neutrophils in marmosets following SARS-CoV-2 challenge. Filled red symbols denote a reduction in HLA-DR expression (defined as 10% below baseline for each individual animal determined from pre-challenge samples); pink open circle denotes a poorly stained sample on day 7 for animal M6 and this data therefore should be treated with caution. (C) The proportion of monocytes in PBMCs from blood collected prior to and post-challenge. The T:B cell ratio (D) in PBMCs from blood collected prior to challenge and on days 1, 2, 3, 4, 7, 14, and 21 post-challenge were defined by size, granularity, and expression of CD3+:CD20+. (E) Proportion of macrophages and neutrophils in marmoset lung following SARS-CoV-2 challenge (n = 2 per time point). Samples from naïve animals (n = 4) were processed during the study to provide resting levels. (F) Quantification of CRP in marmoset plasma detected by ELISA in marmoset plasma samples. Data is presented for each animal. The COVID diagnostic level is the threshold of diagnostically significant levels of CRP described in human COVID-19 cases. In all figures, data is presented for each animal and the normal ranges were defined by the pre-challenge values. Statistically significant differences from pre-challenge values were determined by one-way ANOVA where * p < 0.05, ** p < 0.01 and *** p < 0.001.