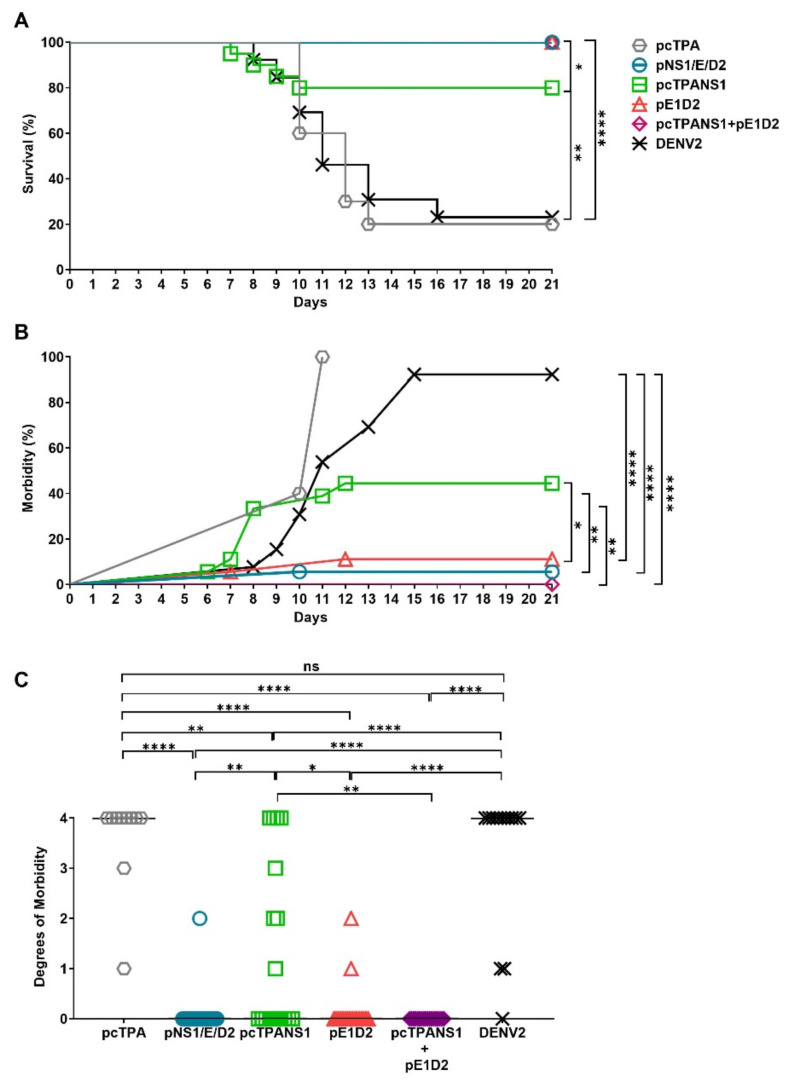
Figure 7.
Protection generated in BALB/c mice immunized with the different DNA vaccines after a lethal challenge with DENV2. Animals were i.m. immunized with the different DNA vaccines and monitored for 21 days after DENV2 inoculation by the i.c. route, for assessment of survival (A) and morbidity (B) rates, and the different morbidity degrees (C). A semi-quantitative analysis of the clinical signs of infection was made using a morbidity degree scale from 0 to 4: 0 = absence of clinical signs; 1 = paralysis in one hind leg or alteration of the spinal column; 2 = severe paralysis in one leg and alterations of the spinal column or severe paralysis on both hind legs; 3 = severe paralysis in the hind legs and alterations of the spinal column; and 4 = death. Lines represent the median of the observed degrees (C). Asterisks indicate significant differences between experimental groups using Log-Rank (Mantel–Cox) test in (A,B) or the non-parametric two-tailed Mann–Whitney statistical test in (C) (* p < 0.05; ** p < 0.01; and **** p < 0.0001), ns: non-significant. Data are representative of two independent experiments (n = 8–10). Significant differences between pcTPA and other experimental groups in (A, B) are the same as for the non-immunized group.