Scheme 1.
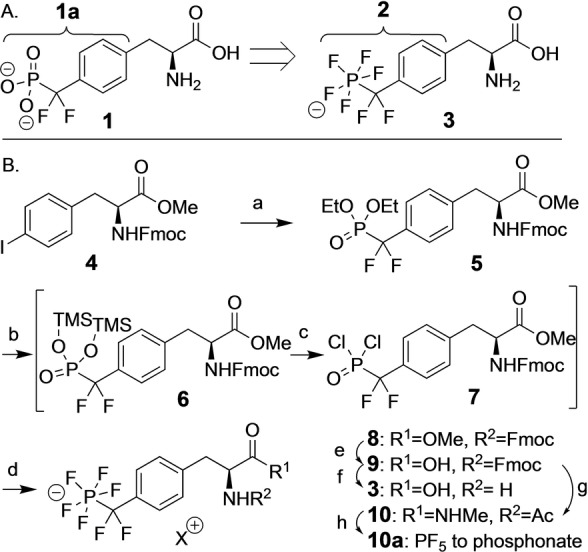
Design (A) and synthesis (B) of PFPDFM‐phenylalanine 3 as a potential phosphotyrosine mimetic. Reaction conditions: a) Cd, CuBr, BrCF2P(O)(OEt)2, DMF, 99 %; b) TMSBr (5 equiv), ACN; c) oxalylchloride (10 equiv), DMF (5 equiv); d) NMe4F (10 equiv) (68 % for b–d); e) bacillus licheniformis protease, 50 mM NH4HCO3 buffer, RT, o.n., 96 %; f) 20 % piperidine in ACN, RT, 8 h, 97 %; g) MeNH3Cl, TBTU, DIPEA, ACN, RT, 1 h, 95 %; 10 % piperidine in ACN, RT, 7 h, 96 %; Ac2O, DIPEA, ACN, RT, 4 h, quant.; h) 2 M HCl, 72 h, quant. TBTU=O‐(Benzotriazol‐1‐yl)‐N,N,N′,N′‐tetramethyluronium tetrafluoroborate, TMSBr=Trimethylsilylbromide, DIPEA=Di‐isopropyl‐ethylamine, RT=room temperature.
