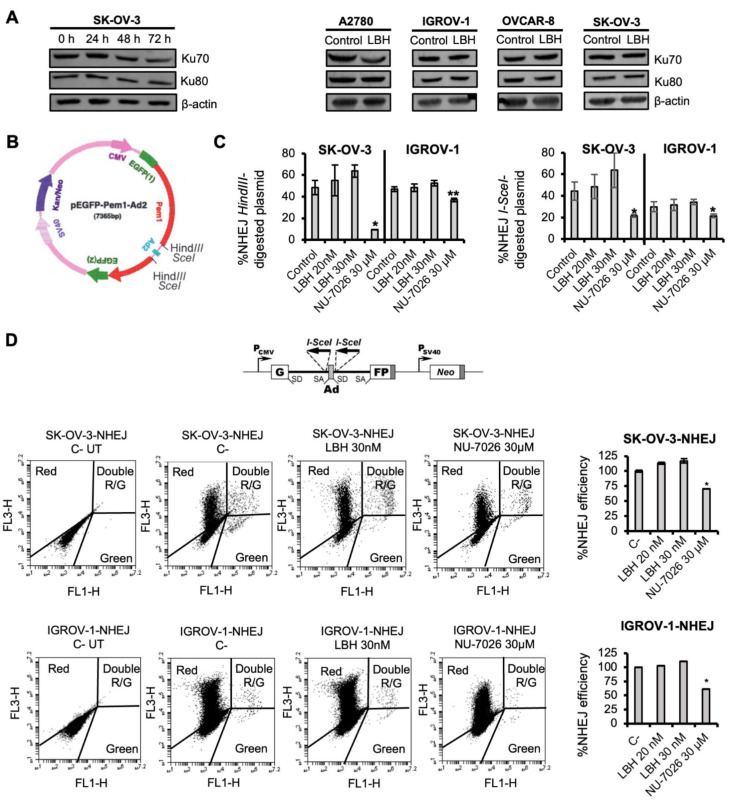
Figure 1.
Panobinostat does not affect DSB repair by NHEJ. (A) Left panel: time-response of NHEJ-related proteins (Ku70, Ku80) after LBH treatment (50 nM) in SK-OV-3 cell line. Right panel: Ku70 and Ku80 expression after 24 h of LBH treatment (20 nM) in OCCLs. β-actin was used as a loading control. (B) Map of pEGFP-Pem1-Ad2. An Ad2 exon is present in the middle of the Pem1 intron, and efficient splicing inactivates the GFP activity and makes the starting substrate GFP-negative. However, both sides of the Ad2 exon present HindII/I-SceI restriction sites. Cleavage with either of these endonucleases removes the Ad exon, and upon successful intracellular plasmid circularization, GFP expression is restored and can be quantified by flow cytometry [47]. (C) Percentage of NHEJ using HindIII- or I-SceI-digested plasmid in IGROV-1 and SK-OV-3 cell lines. Cells were pre-treated or not with the indicated doses of LBH or NU-7026, transfected with the linearized pEGFP-Pem1-Ad2 or supercoiled pEGFP-Pem1 together with the pDSRed plasmid, and incubated again with LBH or NU-7026 for 72 h. The percentage of NHEJ was calculated as described in the Materials and Methods. (D) Top panel: Map of NHEJ-C reporter construct [48]. Bottom panel: Dot plots of nontransfected SK-OV-3 and IGROV-1 cells carrying the NHEJ reporter cassette, and the same cell lines co-transfected with 5 µg of an I-SceI endonuclease-expressing plasmid and 0.5 µg of pDsRed2-N1. The latter were incubated in the presence or absence (C-) of LBH or NU-7026 for an additional 72 h. Correct NHEJ repair restored the GFP gene, which was detected as GFP+ cells. NHEJ efficiency was calculated as the ratio of GFP+ to DsRed+ cells and then normalized to the untreated control. C-: negative control (untreated cells). Data are the mean of three independent experiments. Error bars represent the SD (** p < 0.01, * p < 0.05 compared to controls).