Table 1.
| pH | Ionisation | Stability | Viscosity |
|---|---|---|---|

|
The carboxylate anion primarily exists in its protonated state (COOH). Protonation of the -NH- group of the acetylamine groups may also occur at a pH < 1.6. 
|
At a pH of <2, cleavage of the β-1-3 and β-1-4 glycosidic bonds via acid hydrolysis occurs. This results in the reformation of the individual monosaccharide units coupled with a decrease in molecular mass.
|
A significant decrease in HA viscosity occurs within this pH range. pH-induced degradation of the individual HA chains reduces both the rigidity and the extent of interchain entanglement. Suppression of electrostatic repulsion between the COO- anions contributes to the formation of a more densely compact gel state. Below 1.6, a gel-sol transition occurs due to acetylamino group protonation. |

|
The carboxylate anion exists in its deprotonated state (COO−). Electrostatic repulsion between COO- anions allows for hydrophilic domain expansion. Protonation of the -NH- groups is at a minimum.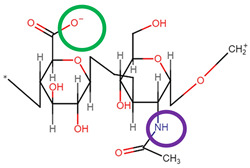
|
The stability of the tertiary β sheet structure is enhanced by the formation of an intermolecular hydrogen bond between the hydroxyl and ether groups  and carboxylate anions and acetylamine groups.  Water molecules can also indirectly facilitate the formation of these hydrogen bonds by behaving as bridging molecules. |
HA exhibits both viscoelastic and pseudoplastic flow behaviour. Increasing shear rate disrupts hydrogen bonding and hydrophobic interactions, resulting in increased chain flexibility and network degradation. This allows the individual chains to align in the direction of the applied flow, leading to a temporary decrease in viscosity. However, HA is also non-thixotropic. Reformation of the network occurs over time upon removal of the shear stress. |

|
At pH > 12, the COO- anions remain in their deprotonated state. The hydroxyl (OH) atoms also exist as alkoxyl anions due to the removal of the hydrogen atoms via excess hydroxide ions.
|
Base-catalysed hydrolysis of the β-1-3 and β-1-4 glycosidic bonds occurs. Due to the deprotonation of the hydroxyl groups, the hydrogen bonds responsible for the stabilisation of the tertiary network begin to degrade.
|
Deformation of the intermolecular hydrogen bonds reduces the rigidity of the backbones of the HA chains. This results in the degradation of the entangled network, coupled with an increase in chain flexibility and mobility. |
