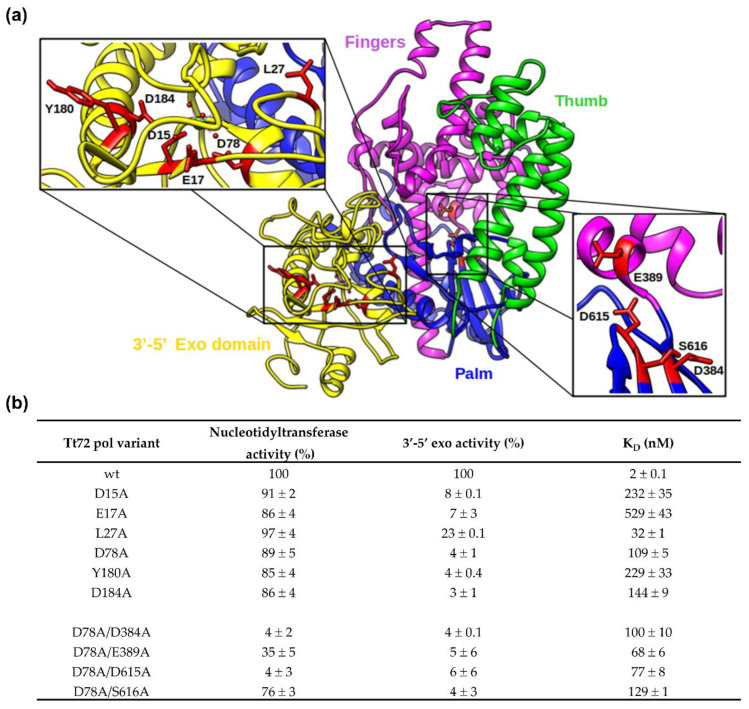
Figure 7.
Structural model of Tt72 DNA polymerase (a). The 3′-5′ exonuclease domain (aa 1-201) is colored yellow. The nucleotidyltransferase domain (aa 202-703) is divided into palm (residues 202-229, 337-365, 376-386, 606-703; colored blue), thumb (residues 230-336, 366-375; green), and fingers (residues 387-605, magenta) subdomains. Additionally, the residues that are crucial for catalytic activity have been zoomed in (D15, E17, D78, D184, Y180 are responsible for exonucleolytic activity—left, and D384, E389, D615, S616 are involved in the template-directed polymerization of dNTPs onto the growing primer strand of duplex DNA—right). (b) Activity and DNA binding affinity (KD) of Tt72 pol and its substitution variants.