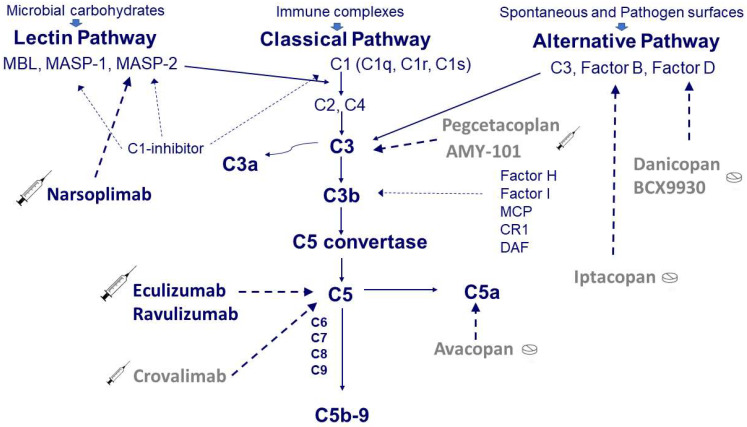
Figure 1.
Simplified scheme of the complement system and target sites of the available drugs. Complement can be activated through three pathways: the classical pathway triggered by antibody-antigen complex, the alternative pathway spontaneously activated at a low level or triggered by specific surface antigens and the lectin pathway activated by binding mannose residues on the pathogen surface. The classical pathway starts from the three components of C1, i.e., C1q and the two proteases C1r and C1s. The activation of C1 in turn induces the activation of C2 and C4, which are also activated by the proteases associated with the mannose-binding lectin (MBL), i.e., MASP-1 and MASP-2. The activation of the classical and lectin pathways is controlled by a C1-inhibitor that can block C1r, C1s, MASP-1, and MASP-2. The alternative pathway, composed of C3, Factor B, and Factor D, is regulated by soluble inhibitors such as factor H and factor I as well as by cell-bound inhibitors such as membrane cofactor protein (MCP), complement receptor 1 (CR1), and decay-accelerating factor (DAF). The activation of the three pathways (classical, lectin, and alternative) converges on the common pathway with the formation of strong inflammatory mediators, such as C3a and C5a, and the production of the C5b-9 membrane attack complex (MAC) that lyses target cells. Therapy with eculizumab blocks C5, whereas therapy with narsoplimab blocks MASP-2. Although never used in TA-TMA, other complement inhibitory drugs are available at different stages of development, such as crovalimab for C5, iptacopan for factor B, danicopan for factor D, and pegcetacoplan for C3.