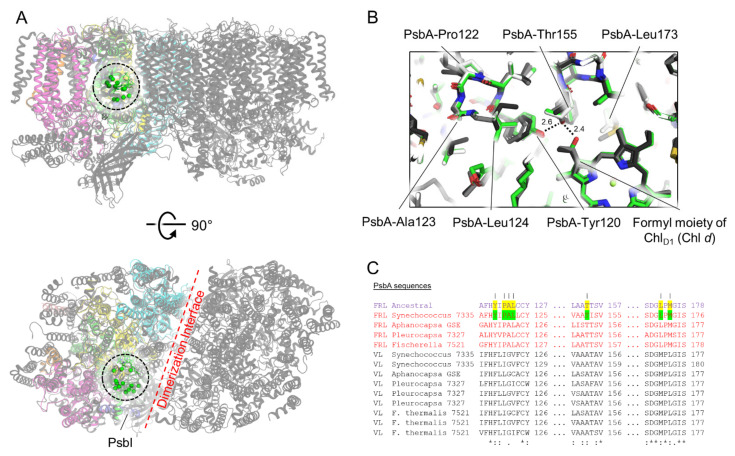
Figure 3.
Conserved ancestral FRL-PsbA residues near the ChlD1 site of the electron transfer chain. In (A), the structure of apo-FRL-PSII from Synechococcus 7335 (colors) is shown superimposed with the structure of the PSII holocomplex from Synechocystis 6803 (grey, PDB 7N8O). The Cα atom from each of the conserved residues in the cluster (black dashed line) near the ChlD1 site is shown as a green sphere. (B) A magnified view of this region. The Synechococcus 7335 apo-FRL-PSII structure (green), the homology model of the FRL-ancestral sequence (white), and two non-FaRLiP holocomplex PSII structures (light and dark grey from T. vulcanus [PDB 3WU2] and Synechocystis 6803, respectively) are superimposed. H-bonding interactions involving the C3 formyl moiety of Chl d in dashed lines with distances in units of Å are also shown. In (C), partial sequence alignments are shown that include the FRL-PsbA ancestral sequence, and FRL- and VL-specific PsbA sequences from extant FaRLiP-capable cyanobacteria. FRL-specific residues conserved in extant cyanobacteria are highlighted green in the sequence from Synechococcus 7335. If the same position is conserved in the FRL ancestral sequence, it is highlighted yellow. Vertical lines above residue positions in (C) correspond to amino acids from the Synechococcus 7335 apo-FRL-PSII structure labeled in (B). The Clustal Omega sequence conservation identifiers are shown below the alignment.