Figure 5.
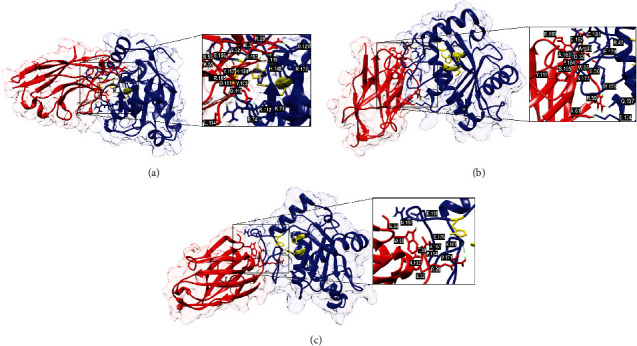
Molecular docking results showing the binding sites of VHHs (VHH47, VHH61, and VHH64) on the surface of BjussuMP-II. Cartoon representations of the BjussuMP-II VHH interaction structures (side view) covered by a translucent electronic surface, where the α-chains of BjussuMP-II are shown as blue ribbons and the VHH is in red. The active site of BjussuMP-II (His144, His148, His154, and Glu145) is represented in yellow. (a) Anti-BjussuMP-II VHH47 and BjussuMP-II. (b) Anti-BjussuMP-II VHH61 and BjussuMP-II. (c) Anti-BjussuMP-II VHH64 and BjussuMP-II. The interaction sites have been magnified to show the hydrogen bonds that formed between the amino acid residues.
