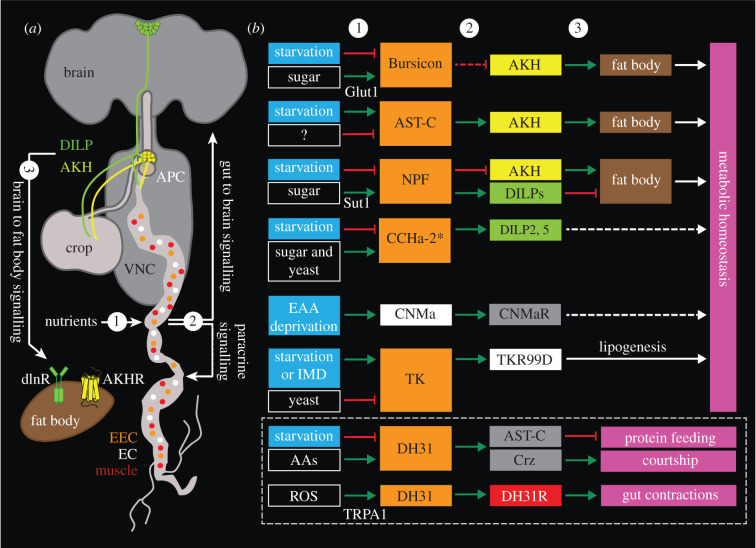
Figure 9.
Interorgan peptide signalling: brain, intestine and fat body. Peptides from the gut modulate metabolic homeostasis, behaviours and gut physiology via state-dependent (mainly nutritional) gut-to-brain and paracrine signalling. See electronic supplementary material, figure S5 for distribution of gut peptides. (a) A schematic showing the signalling pathways mediating the effects of peptides produced by gut enteroendocrine cells (EECs; orange) and enterocytes (ECs; white). (1) These cells predominantly sense nutrients such as carbohydrates, yeast and amino acids, but they can also sense ROS, and receive inputs from the innate IMD pathway. (2) Once these cells are activated, they release peptides into the circulation for local effects (paracrine signalling) on gut enterocytes or muscles (red). In addition, the peptides target the nervous system, including the adipokinetic hormone (AKH)-producing cells (APCs) and Drosophila insulin-like peptide (DILP)-producing cells (IPCs). (3) AKH and DILPs in turn target their receptors (AKHR and dInR, respectively) on the fat body to influence metabolic homeostasis. (b) Various intestinal peptidergic pathways influence metabolic homeostasis in a feeding-state-dependent manner. Sugar activates Bursicon EECs via the Glut1 glucose transporter and Bursicon indirectly inhibits APCs to regulate lipid homeostasis [241]. Starvation triggers release of Allatostatin-C (AstC), which activates APCs to promote energy mobilization [242]. Neuropeptide F (NPF)-producing EECs respond to sugar via the Sut1 transporter, and released NPF inhibits APCs and activates IPCs to regulate lipid metabolism [243]. Sugars and yeast activate, while starvation inhibits release of CCHa-2 from cells in the gut and fat body. CCHa-2 stimulates release of DILP2 and 5 to influence organismal growth [244]. Deprivation of EAAs activates CNMa-producing ECs, to regulate metabolic homeostasis via actions on CNMaR-expressing neurons [245]. Both starvation and IMD pathway activate, whereas yeast inhibits tachykinin (TK)-producing EECs [161,162]. TK acts locally on enterocytes via its receptor TKR99D to affect lipogenesis. Amino acids activate diuretic hormone 31 (DH31) producing EECs [45]. DH31 regulates the balance between feeding and courtship via actions on AstC- and corazonin (CRZ)-producing neurons, respectively [39]. Moreover, DH31 EECs can also sense reactive oxygen species (ROS) via the TRPA1 receptor to regulate gut contractions [246]. Boxes in (b) are colour-coded to match the cell types in (a). Dashed arrows indicate indirect actions. The two receptors in the fat body were generated in BioRender.