FIGURE 1.
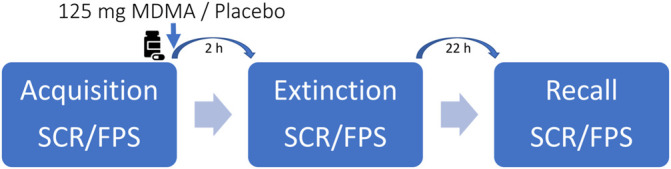
Schematic study day. Participants underwent every two sessions with either 125-mg 3,4-methylenedioxymethamphetamine (MDMA) or placebo. The order was randomized but balanced. The washout period between the study days was at least 30 days. Autonomic and subjective effects were assessed throughout the study day. Blood samples for MDMA and oxytocin blood plasma concentrations were collected before and periodically up to 24 h after drug ingestion.
