Abstract
BACKGROUND
The value of neoadjuvant radiotherapy following (m)FOLFIRINOX for patients with borderline resectable (BR) pancreatic ductal adenocarcinoma (PDAC) is uncertain.
METHODS
We conducted an international retrospective cohort study including consecutive patients with BR PDAC who received (m)FOLFIRINOX as initial treatment (2012–2019) from the Trans-Atlantic Pancreatic Surgery Consortium. Since the decision for radiotherapy is made after chemotherapy, patients with metastases or deterioration after (m)FOLFIRINOX or a performance score ≥2 were excluded. Patients who received radiotherapy following (m)FOLFIRINOX were matched 1:1 by nearest neighbor propensity scores with patients who did not. Propensity scores were calculated using sex, age (≤70 versus >70), performance score (0 versus 1), tumor size (0–20 versus 21–40 versus >40mm), tumor location (head/uncinate versus body/tail), number of cycles (1–4 versus 5–8 versus >8), and baseline carbohydrate antigen (CA) 19–9 (≤500 versus >500 U/mL). Primary outcome was overall survival (OS) from diagnosis.
RESULTS
Of 531 patients who received neoadjuvant (m)FOLFIRINOX for BR PDAC, 424 met inclusion criteria and 300 (70.8%) were propensity score matched. After matching, median OS was 26.2 months (95% confidence interval [CI]: 24.0–38.4) with radiotherapy versus 32.8 months (95% CI: 25.3–42.0) without radiotherapy (p=0.71). Radiotherapy was associated with a lower resection rate (55.3% versus 72.7%, p=0.002). In patients who underwent a resection, radiotherapy was associated with a comparable margin-negative resection rate (>1mm) (70.6% versus 64.8%, p=0.51), more node-negative disease (57.3% versus 37.6%, p=0.01), and more major pathologic response with <5% tumor viability (24.7% versus 8.3%, p=0.006). The OS of conventional and stereotactic body radiation approaches was similar (median OS: 25.7 versus 26.0 months, p=0.92).
CONCLUSIONS
In patients with BR PDAC, neoadjuvant radiotherapy following (m)FOLFIRINOX was associated with more node-negative disease and better pathologic response in patients who underwent resection, yet no difference in OS was found. Routine use of radiotherapy cannot be recommended based on these data.
Keywords: pancreatic neoplasms* / therapy, radiation, propensity score, pancreatectomy, survival analysis
Table of Contents:
Neoadjuvant radiotherapy following (m)FOLFIRINOX was not associated with improved OS in patients with BR PDAC. Routine use of radiotherapy cannot be recommended based on these data.
Lay summary:
In this international retrospective cohort study, 150 patients with BR PDAC who received radiotherapy following (m)FOLFIRINOX were 1:1 propensity score matched to 150 patients who received (m)FOLFIRINOX alone. After matching, median OS was 26.2 months (95% CI: 24.0–38.4) with radiotherapy versus 32.8 months (95% CI: 25.3–42.0) without radiotherapy (p=0.71). Radiotherapy was associated with fewer resections (55.3% vs. 72.7%, p=0.002), comparable margin-negative resection rates (70.6% vs. 64.8%, p=0.51), more node-negative disease (57.3% vs. 37.6%, p=0.01), and more major pathologic response (24.7% vs. 8.3%, p=0.006). In conclusion, neoadjuvant radiotherapy following (m)FOLFIRINOX was not associated with improved OS in patients with BR PDAC.
1. INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) represents one of the most aggressive solid tumors. Localized PDAC is classified into radiographic stages as potentially resectable (PR), borderline resectable (BR), or locally advanced (LA) disease, based on the extent of venous and arterial involvement.1,2 Although several staging criteria are currently used, patients with BR PDAC are generally considered technically resectable, but with increased risk of a microscopic margin-positive (R1) resection. The National Comprehensive Cancer Network (NCCN) guideline recommends neoadjuvant therapy for patients with BR PDAC to increase the likelihood of a microscopically radical (R0) resection.2 Moreover, a neoadjuvant approach allows for early treatment of occult micro-metastatic disease and ensures systemic treatment for all patients without the risk of postoperative complications precluding adjuvant treatment.3 Last, it allows tumor biology to declare itself for patients with elevated tumor markers, thereby improving patient selection for surgery.4
In the current NCCN guideline, neoadjuvant chemotherapy may be followed by radiotherapy, without clear specification on when this may be considered.2 Cohort studies reported that neoadjuvant radiotherapy is associated with better locoregional control compared with chemotherapy alone. However, a benefit in overall survival (OS) has not been clearly demonstrated.5–8 The long-term results of the PREOPANC trial found better OS with neoadjuvant chemoradiotherapy compared with upfront surgery in patients with BR and PR PDAC.9,10 However, this study did not directly compare neoadjuvant chemotherapy with or without radiation. Moreover, the PREOPANC trial used gemcitabine alone that was shown inferior to FOLFIRINOX (i.e. 5-fluorouracil with leucovorin, irinotecan, and oxaliplatin) in the metastatic and adjuvant setting.11,12 By extrapolation of these results, the NCCN guideline has included neoadjuvant (m)FOLFIRINOX as one of the preferred first-line treatments for patients with BR PDAC with a good performance status.2 Several retrospective studies have already shown promising results using neoadjuvant (m)FOLFIRINOX with or without additional radiotherapy.13–16
This study aimed to assess the effectiveness of neoadjuvant radiotherapy following (m)FOLFIRINOX in patients with BR PDAC. In the absence of published phase III trials, we performed propensity score matched analysis of a large observational cohort to minimize known confounding biases.17
2. METHODS
2.1. Study design and patients
The international Trans-Atlantic Pancreatic Surgery (TAPS) Consortium includes five PDAC referral centers from the United States (University of Pittsburgh Medical Center; MD Anderson Cancer Center; Memorial Sloan Kettering Cancer Center) and the Netherlands (Amsterdam UMC; Erasmus MC University Medical Center). All participating centers obtained ethical approval from local Institutional Review Boards. Due to the retrospective nature of the study, the requirement to obtain informed consent was waived. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline, modified for reporting propensity score analysis.17
The consortium centers aggregated a consecutive cohort of patients diagnosed with clinically localized PDAC between 2012 and 2019, who started with (m)FOLFIRINOX as initial treatment. Radiographic stage was based on the MDACC classification system4 or the NCCN criteria applicable at time of diagnosis (the other four centers). For patients from the Netherlands, stage according to NCCN criteria was reconstructed based on the exact extent of vascular contact with and possible occlusion of surrounding vasculature after radiologic review of the CT scan prior to start of treatment.
For the present study, all patients diagnosed with BR PDAC were identified from the TAPS total cohort of 1835 patients. Since the decision for radiotherapy is generally made after completion of chemotherapy, patients were excluded in case of metastatic disease or clinical decline at restaging following (m)FOLFIRINOX, or in case of a baseline World Health Organization (WHO) performance score of ≥2. Furthermore, patients were excluded if it was unknown whether they had received neoadjuvant radiotherapy. The decision to proceed with and the type of neoadjuvant radiotherapy was based on the discussions at each institution’s local multidisciplinary meeting. Radiotherapy options included conventional regimens (typically 30 Gy in 10 fractions or 50.4 Gy in 28 fractions, often with concurrent chemotherapy) or stereotactic body radiation therapy (SBRT) regimens of ≥ 5 Gy per fraction in 5 fractions.
2.2. Data collection and definitions
Prespecified data on patient demographics, tumor characteristics, treatment details, and clinical and pathological outcomes were collected locally and merged after de-identification. OS was defined from date of tissue diagnosis to date of death, with censoring at the date of last follow-up for patients with no event. The date of final analysis for the cohort was December 31st, 2020. The 8th edition of the American Joint Committee on Cancer Staging (AJCC) Manual was used for tumor-node-metastasis (TNM) staging,18 the 1mm definition for resection margin status,19 and pathologic response was categorized as major/complete (<5% tumor viability) or not (≥5%).20 One biweekly treatment of (m)FOLFIRINOX was considered one cycle.
2.3. Statistical analysis
Clinicopathological characteristics were presented based on treatment (radiotherapy vs. no radiotherapy) using descriptive statistics. Chi-square test was used to compare categorical variables and the Mann-Whitney U test for continuous variables. To minimize confounding biases, propensity score matching was performed using 1:1 nearest neighbor matching. Propensity scores were calculated using a logistic regression model including known prognostic factors that may determine subsequent treatment; sex, age at diagnosis (≤70 vs. >70 years), performance score (WHO 0 vs. WHO 1), tumor size (0–20 vs. 21–40 vs. >40 mm), tumor location (head/uncinate vs. body/tail), baseline CA 19–9 (≤500 vs. >500 U/mL), and number of neoadjuvant (m)FOLFIRINOX cycles (1–4 vs. 5–8 vs. >8). Sampling without replacement was used and only patients with complete data on the matching factors were included. After matching, a standardized difference of <0.10 was considered an insignificant and acceptable imbalance.21,22 The primary endpoint was OS for the matched cohort, assessed using Kaplan-Meier estimates. The difference in OS between the treatment groups was tested using the log-rank test. The treatment effect was estimated using a Cox proportional hazards model and expressed as a hazard ratio (HR) with corresponding 95% confidence interval (CI). Secondary endpoints included differences in pathological outcomes between the matched treatment groups.
A subgroup analysis separately evaluated patients from the matched cohort who did or did not undergo a resection, comparing the treatment groups. A second subgroup analysis compared patients receiving conventional radiotherapy and SBRT.
All tests were two-sided and a p-value <0.05 was considered statistically significant. Analyses were performed using R software, version 3.4.3. The MatchIt package was used to create the matched sample.
3. RESULTS
3.1. Patient and treatment characteristics
Between 2012 and 2020, 531 patients with BR PDAC who received at least one cycle of neoadjuvant (m)FOLFIRINOX as initial treatment were extracted from the total TAPS cohort of 1835 patients. Of those, 107 patients (20.2%) were excluded for reasons shown in Figure 1. Of the remaining 424 patients, 195 (46.0%) received neoadjuvant radiotherapy. Overall, patients received a median of six cycles (IQR 4–8) of neoadjuvant (m)FOLFIRINOX (Table 1).
Figure 1.

Flow diagram of patient enrollment.
Table 1.
Baseline characteristics and treatment details, unmatched and matched cohort.
| Overall | No radiotherapy | Radiotherapy | P-value | Overall | No radiotherapy | Radiotherapy | P-value |
|---|---|---|---|---|---|---|---|
| n = 424 | n = 229 | n = 195 | n = 300 | n = 150 | n = 150 | ||
| 189 (44.6) | 92 (40.2) | 97 (49.7) | 0.06 | 147 (49.0) | 72 (48.0) | 75 (50.0) | 0.82 |
| 64 [57, 70] | 64 [58, 69] | 64 [57, 70] | 0.85 | 64 [57, 70] | 65 [58, 70] | 64 [57, 70] | 0.57 |
| <0.001* | 0.91 | ||||||
| 222 (52.4) | 139 (60.7) | 83 (42.6) | 150 (50.0) | 76 (50.7) | 74 (49.3) | ||
| 202 (47.6) | 90 (39.3) | 112 (57.4) | 150 (50.0) | 74 (49.3) | 76 (50.7) | ||
| 26 [23, 29] | 26 [23, 30] | 26 [24, 29] | 0.78 | 26 [24, 30] | 26 [23, 30] | 26 [24, 29] | 0.77 |
| 335 (79.0) | 184 (80.3) | 151 (77.4) | 0.54 | 229 (76.3) | 117 (78.0) | 112 (74.7) | 0.59 |
| 34 [26, 41] | 33 [26, 41] | 34 [27, 43] | 0.29 | 34 [27, 41] | 34 [26, 41] | 34 [27, 41] | 0.58 |
| 196 [48, 653] | 178 [42, 578] | 232 [75, 706] | 0.13 | 198 [46, 653] | 157 [30, 582] | 238 [73, 710] | 0.14 |
| 0.001* | 0.88 | ||||||
| 142 (33.5) | 95 (41.5) | 47 (24.1) | 92 (30.7) | 48 (32.0) | 44 (29.3) | ||
| 230 (54.2) | 109 (47.6) | 121 (62.1) | 172 (57.3) | 84 (56.0) | 88 (58.7) | ||
| 52 (12.3) | 25 (10.9) | 27 (13.8) | 36 (12.0) | 18 (12.0) | 18 (12.0) |
significant p-value <0.05.
Abbreviations: BMI, body mass index; CA 19-9, carbohydrate antigen 19-9; CT, computed tomography; IQR, interquartile range; n, number; WHO, World Health Organization. Missing data: BMI (n=6), tumor size (n=12), CA 19-9 (n=31).
3.2. Radiotherapy regimens
Of the 195 patients with BR PDAC who received neoadjuvant radiotherapy, 128 patients (65.6%) received conventional radiotherapy and 63 patients (32.3%) received SBRT. For four patients, radiotherapy treatment specifics were unknown. For the patients receiving conventional radiotherapy, concurrent chemotherapy was given as radiosensitizer in 115/128 patients (89.8%) (Supplementary Table 1).
3.3. Propensity score matching
Baseline characteristics and treatment details before and after propensity score matching are summarized in Table 1. Before matching, patients in the radiotherapy group had worse performance scores (p<0.001) and received more neoadjuvant cycles of (m)FOLFIRINOX (p=0.001). With propensity score matching, 150 patients from the radiotherapy group (77%) were matched to 150 patients from the no radiotherapy group (66%). After matching, the absolute standardized differences for the unbalanced variables were low (range 1–5%), resulting in comparable patient, tumor, and treatment characteristics.
3.4. Survival analysis
After a median follow-up time of 36.5 months, 253/424 patients (59.7%) had died. The median OS in the unmatched cohort was 25.7 months (95% CI: 23.7–31.8) with radiotherapy versus 29.1 months (95% CI: 23.2–35.0) without radiotherapy (HR 0.99, 95% CI: 0.77–1.26, p=0.91) (Figure 2a). After matching, the median OS was 26.2 months (95% CI: 24.0–38.4) with radiotherapy versus 32.8 months (95% CI: 25.3–42.0) without radiotherapy (HR 1.06, 95% CI: 0.78–1.43, p=0.71) (Figure 2b). The 5-year OS was comparable (27 vs. 26%).
Figure 2. Overall survival from diagnosis for patients who did or did not receive neoadjuvant radiotherapy after (m)FOLFIRINOX, (a) in the unmatched cohort, (b) in the propensity score matched cohort, (c) in the propensity score matched cohort for patients who did or did not undergo a resection.
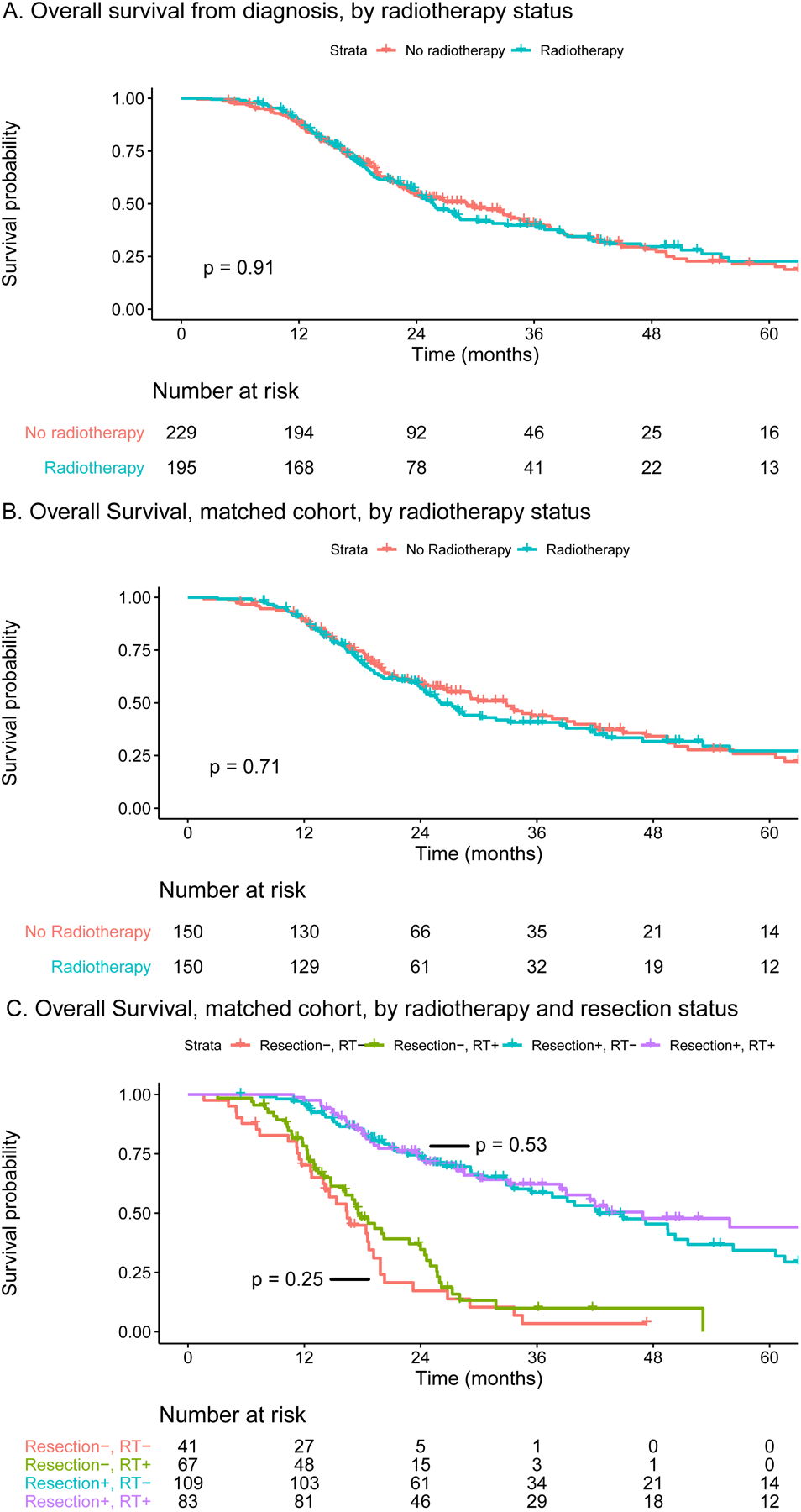
One-to-one matching based on sex, age at diagnosis (≤70 vs. >70 year), performance score (WHO 0 vs. WHO 1), tumor size (0–20 vs. 21–40 vs. >40 mm), tumor location (head/uncinate vs. body/tail), baseline CA 19–9 (≤500 vs. >500), and number of neoadjuvant cycles of (m)FOLFIRINOX (1–4 vs. 5–8 vs. >8).
Abbreviations: CA, carcinogen antigen; RT, radiotherapy; WHO, World Health Organization.
3.5. Surgical exploration and resection in the matched cohort
At multidisciplinary evaluation following completion of (m)FOLFIRINOX and radiotherapy in the radiotherapy group, 30 patients (20.0%) had developed locally unresectable disease, 19 patients (12.7%) with metastatic disease that became manifest at restaging following radiotherapy, and 2 patients (1.3%) had clinically declined precluding surgery. In the no radiotherapy group, 15 patients (10.0%) had developed locally unresectable disease after completion of (m)FOLFIRINOX alone. As noted, patients with metastatic disease at restaging following (m)FOLFIRINOX were already excluded from the analyses.
Surgical exploration was recommended for the remaining 99 patients (66.0%) in the radiotherapy group and 135 patients (90.0%) in the no radiotherapy group (p<0.001). The median time from diagnosis to surgery was 229 days (IQR 189 – 268) in the radiotherapy group and 146 days (IQR 125 – 175) in the no radiotherapy group (p<0.001). In total, 83 patients (55.3%) underwent a resection in the radiotherapy group versus 109 patients (72.7%) in the no radiotherapy group (p=0.002). The resection rate of patients recommended for surgery was comparable (83.8% vs. 80.7%, p=0.54). A vascular resection was performed in 43 patients (51.8%) in the radiotherapy group versus 45 patients (42.1%) in the no radiotherapy group (p=0.23). Only one patient died within 30-days following resection, who was included in the no radiotherapy group. Adjuvant chemotherapy was started in 33 patients (39.8%) in the radiotherapy versus 85 patients (78.0%) in the no radiotherapy group (p<0.001). Palliative treatment was started in a comparable number of patients (52.0% vs. 51.3%, p=0.62).
Figure 2c shows the OS curves for both treatment groups, separately for the resection and non-resection cohort. For patients who underwent a resection, the median OS was 46.9 months (95% CI: 38.4–83.9) with radiotherapy versus 42.3 months (95% CI: 35.4–56.2) without radiotherapy (HR 0.87, 95% CI: 0.58–1.32, p=0.53). With resection, the 5-year OS was 44% (95% CI: 32–61%) with radiotherapy versus 34% (95% CI: 24–49%) without radiotherapy. For patients who did not undergo a resection, the median OS was 17.5 months (95% CI: 16.0–24.4) with radiotherapy versus 16.4 months (95% CI: 13.9–19.8) without radiotherapy (HR 0.77, 95% CI: 0.49–1.20, p=0.25). Without resection, the 5-year OS was 10% (95% CI: 4–26%) with radiotherapy versus 3% (95% CI: 1–24%) without radiotherapy.
3.6. Pathological outcomes in the matched cohort
Patients in the radiotherapy group had a similar R0 resection rate (70.6% vs. 64.8%, p=0.53), more node-negative disease (ypN0: 57.3% vs. 37.6%, p=0.01), and more often had a major or complete pathologic response (24.7% vs. 8.3%, p=0.01) (Table 2).
Table 2.
Pathological outcomes of patients who underwent a resection in the matched cohort.
| Overall | No radiotherapy | Radiotherapy | P-value |
|---|---|---|---|
| n = 192 | n = 109 | n = 83 | |
| 25 [18, 33] | 25 [20, 30] | 25 [17, 36] | 0.83 |
| 0.13 | |||
| 8 (4.2) | 2 (1.8) | 6 (7.3) | |
| 145 (75.9) | 87 (79.8) | 58 (70.7) | |
| 38 (19.9) | 20 (18.3) | 18 (22.0) | |
| 0.01* | |||
| 88 (46.1) | 41 (37.6) | 47 (57.3) | |
| 67 (35.1) | 41 (37.6) | 26 (31.7) | |
| 36 (18.8) | 27 (24.8) | 9 (11.0) | |
| 0.53 | |||
| 118 (67.0) | 70 (64.8) | 48 (70.6) | |
| 58 (33.0) | 38 (35.2) | 20 (29.4) | |
| 0.01* | |||
| 5 (2.9) | 5 (5.0) | 0 (0.0) | |
| 125 (72.3) | 77 (77.0) | 48 (65.8) | |
| 43 (24.9) | 18 (18.0) | 25 (34.2) | |
| 147 (77.4) | 84 (77.8) | 63 (76.8) | 1 |
| 101 (53.4) | 64 (59.3) | 37 (45.7) | 0.09 |
| 0.01* | |||
| 28 (15.8) | 8 (8.3) | 20 (24.7) | |
| 149 (84.2) | 88 (91.7) | 61 (75.3) |
8th edition of American Joint Committee on Cancer Staging.
1mm definition of Royal College of Pathologists.
significant p-value <0.05. Abbreviations: G, grade; IQR, interquartile range; n, number; yp, pathological outcome after neoadjuvant treatment. Missing data: tumor size (n=2), ypT (n=1), ypN (n=1), resection margin (n=16), tumor differentiation (n=19), perineural invasion (n=2), lymphovascular invasion (n=3), pathologic response (n=15)
3.7. Conventional radiotherapy versus SBRT
The median OS was 26.0 months (95% CI: 22.4–42.0) for the 63 patients receiving SBRT versus 25.7 months (95% CI: 22.5–38.4) for the 128 patients receiving conventional radiotherapy (HR 1.02, 95% CI: 0.69–1.52, p=0.92) (Figure 3).
Figure 3.

Overall survival from diagnosis for patients with BR PDAC who received neoadjuvant radiotherapy after (m)FOLFIRINOX, comparing stereotactic body radiation therapy (SBRT) with conventional radiotherapy (RT).
4. DISCUSSION
This multicenter propensity score matched analysis of 300 patients with BR PDAC who received (m)FOLFIRINOX as initial treatment showed a median OS of 26.2 months with radiotherapy compared with 32.8 months without radiotherapy (HR 1.06, 95% CI: 0.78–1.43, p=0.71). In addition, no difference in survival was found between the treatment groups when separately analyzing the resection and non-resection cohort. In those patients who underwent surgical resection, neoadjuvant radiotherapy was associated with more node-negative disease and better pathologic response. The OS of conventional and stereotactic body radiation approaches was similar.
To date, only one randomized phase II trial has been presented directly comparing neoadjuvant multi-agent chemotherapy with or without radiotherapy.23,24 The ALLIANCE A021501 trial compared neoadjuvant mFOLFIRINOX (8 cycles) to mFOLFIRINOX (7 cycles) followed by SBRT (33–40 Gy in 5 fractions) or HIGRT (25 Gy in 5 fractions). After inclusion of 56 patients, the radiotherapy arm was closed due to futility regarding the R0 resection rate. At final analysis, OS in the radiotherapy arm (median OS: 17.1 months) was not better compared to historical data (18–23 months) and lower compared to mFOLFIRINOX without radiotherapy (31.0 months). Median OS without radiotherapy was similar between the ALLIANCE trial and the present study. In the ALLIANCE trial, SBRT rather than conventional RT was used, based on promising results in patients with LA PDAC.25–27 In the present study, we found similar survival between SBRT and conventional radiotherapy for BR PDAC.
In a meta-analysis including 512 patients with BR or PR PDAC from 15 small single arm studies, neoadjuvant radiotherapy following (m)FOLFIRINOX was not associated with a difference in OS.28 Retrospective series evaluating neoadjuvant chemotherapy regimens other than (m)FOLFIRINOX5–8 and the randomized LAP-07 trial for patients with locally advanced PDAC29 also found no difference in OS with and without radiotherapy. Four studies found better survival with neoadjuvant radiotherapy following multi-agent chemotherapy regimens.16,30–32 Three of these four studies, however, only included the selected subgroup of patients who underwent a resection, thereby introducing selection bias. In the no radiotherapy group, a patient who undergoes a resection might be diagnosed with liver metastases three months after surgery; in the radiotherapy group, the same patient would be diagnosed with liver metastases at restaging after radiotherapy and would therefore not end up in the resection cohort. We found that 12.7% of patients in the radiotherapy group had developed metastatic disease at restaging after radiotherapy, illustrating this selection bias in studies that only report the cohort who underwent a resection. These patients had an additional period for metastatic disease to become overt at restaging after radiotherapy. Consequently, a resection is avoided in the radiotherapy group in about 1 in 8 patients who would have developed early recurrent disease without a period of radiotherapy. In the present study, patients in the radiotherapy group also had higher risk of locally advanced (i.e., unresectable) disease at radiologic restaging (20.0% vs. 10.0%). Despite propensity matched analysis, patients in the radiotherapy group may have had more extensive vascular involvement at baseline within the spectrum of BR PDAC or less local response to (m)FOLFIRINOX (i.e., residual confounding).
In patients who underwent a resection in the matched cohort, radiotherapy was associated with a higher frequency of node-negative disease and major pathologic response, which is consistent with literature.5–7,30,31,33 This may be explained by the locoregional effect of radiotherapy, although it may also be partly explained by selecting out patients with progressive disease during the prolonged treatment time for radiotherapy. No difference in R0 resection rate was found between the radiotherapy and no radiotherapy group. Other studies show conflicting data on this outcome.6,7,24,28,30,31 Differences in the definition of R0 and pathology grossing techniques hamper the comparability of margin status across studies.19,34,35 Of note, the conventional definition of an R0 resection based on 1 mm clearance may not be adequate following neoadjuvant therapy due to its cytoreductive effect, although consensus on the optimal assessment of margin status in this setting is lacking.36 Since the main effect of radiotherapy seems to be improved locoregional control, future studies should try to identify those patients for whom survival is mainly defined by their local tumor.
Some surgeons have raised concerns that preoperative radiotherapy may increase postoperative complications. Two recent studies, however, have found no difference in postoperative complications between patients with and without preoperative radiotherapy. Moreover, the rate of postoperative pancreatic fistula was lower in patients who received preoperative radiotherapy.37,38
Currently, three randomized trials assess the role of neoadjuvant radiotherapy for BR PDAC. The 3-arm BRPCNCC-1 trial compares neoadjuvant gemcitabine plus nab-paclitaxel with or without SBRT to S1 plus nab-paclitaxel with SBRT in 150 patients.39 The PANDAS-PRODIGE44 trial (NCT02676349) compares neoadjuvant mFOLFIRINOX with or without conventional chemoradiotherapy (50.4 Gy in 28 fractions) in 90 patients. Last, the PREOPANC-2 trial compares neoadjuvant FOLFIRINOX to neoadjuvant gemcitabine-based chemoradiotherapy in 368 patients with BR and PR PDAC.40 It is unlikely, however, that these studies will completely resolve the debate on the added value of neoadjuvant radiotherapy for BR PDAC. Only a large randomized controlled trial (i.e. 500–1000 patients) directly comparing multi-agent systemic treatment with or without radiotherapy could definitively adjudicate whether the improved locoregional control of radiotherapy translates into a clinically relevant survival benefit.
Within the context of these data, routine use of radiotherapy for all BR PDAC patients may not be justified. Improved pathology outcomes in the radiotherapy group suggest that radiotherapy can benefit a subgroup of patients, but this subgroup remains to be identified. Selected radiotherapy prior to surgery may be indicated in patients with threatened margins or for vascular preservation to avoid the need for arterial resection.
The findings reported in this study should be interpreted with some limitations in mind. First, confounding by indication may have occurred, with more advanced tumors (within the definition of BR PDAC) in the radiotherapy group. On the other hand, guarantee-time bias was an advantage for the radiotherapy group.41 These biases were addressed with propensity score matched analysis, but residual bias from unmeasured factors may still be present. Second, data on the exact extent of vascular involvement within the spectrum of BR PDAC and data on disease recurrence (i.e. locoregional or distant) were not available. Last, treatment protocols (e.g., selection for radiotherapy, type of radiotherapy, and subsequent adjuvant and palliative treatment) differed across centers and over time. However, a cohort in which similar patients received different treatments is a requirement for propensity score matching. Moreover, this reflects real-world protocol variations in experienced treatment centers. Strengths of this study include the large sample size, the uniform use of (m)FOLFIRINOX chemotherapy, and the inclusion of patients from experienced referral centers from two different countries.
In conclusion, neoadjuvant radiotherapy following (m)FOLFIRINOX for BR PDAC was not associated with improved OS despite some benefits in node-negative disease and pathologic response in those patients who underwent surgical resection. Routine use of neoadjuvant radiotherapy for all patients cannot be recommended based on these data. Future studies are needed to assess whether specific subgroups of patients with BR PDAC would benefit from neoadjuvant radiotherapy.
Supplementary Material
Acknowledgements:
We would like to acknowledge the Living With Hope Foundation, the Onno Ruding Foundation, the Dutch Cancer Society, and ZonMw for their financial support. In addition, we thank Caitlin McIntyre, Sarah McIntyre, Crisanta Ilagan, and Dana Haviland for helping with the data collection.
Funding:
This study was supported by the Living With Hope Foundation, the Onno Ruding Foundation, the Dutch Cancer Society (10955), and ZonMw (843004108). No funding agency was involved in the design of the study, the data collection, the data analysis, or the interpretation of the data.
List of abbreviations
- AJCC
American Joint Committee on Cancer
- BR
Borderline resectable
- CA 19-9
Carbohydrate antigen 19-9
- CI
Confidence interval
- (m)FOLFIRINOX
5-Fluorouracil with leucovorin, oxaliplatin, and irinotecan, with or without dose modifications
- HIGRT
Hypofractionated image guided radiation therapy
- HR
Hazard ratio
- IQR
Interquartile range
- MDACC
MD Anderson Cancer Center
- NCCN
National Comprehensive Cancer Network
- PDAC
Pancreatic ductal adenocarcinoma
- SBRT
Stereotactic body radiation therapy
- TAPS
Trans-Atlantic Pancreatic Surgery
- TNM
Tumor, node, metastasis
- WHO
World Health Organization
Footnotes
Conflict of interest: EO: Genentech/Roche, Celgene/BMS, BioNTech, BioAtla, AstraZeneca, Arcus, Elicio, Parker Institute, AstraZeneca, Pertzye (funding to MSKCC); Cytomx Therapeutics (DSMB), Rafael Therapeutics (DSMB), Sobi, Silenseed, Tyme, Seagen, Molecular Templates, Boehringer Ingelheim, BioNTech, Ipsen, Polaris, Merck, IDEAYA, Cend, AstraZeneca, Noxxon, BioSapien, Cend Therapeutics (consulting); Bayer (spouse), Genentech-Roche (spouse), Celgene-BMS (spouse), Eisai (spouse). CWT: PanTher Therapeutics (consulting). AW: Histosonics (consulting), AstraZeneca (consulting), Intuitive (travel). For the remaining authors none were declared.
References
- 1.Isaji S, Mizuno S, Windsor JA, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18(1):2–11. [DOI] [PubMed] [Google Scholar]
- 2.Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(4):439–457. [DOI] [PubMed] [Google Scholar]
- 3.Janssen QP, O’Reilly EM, van Eijck CHJ, Groot Koerkamp B. Neoadjuvant Treatment in Patients With Resectable and Borderline Resectable Pancreatic Cancer. Front Oncol. 2020;10:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206(5):833–846; discussion 846–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lutfi W, Talamonti MS, Kantor O, et al. Neoadjuvant external beam radiation is associated with No benefit in overall survival for early stage pancreatic cancer. Am J Surg. 2017;213(3):521–525. [DOI] [PubMed] [Google Scholar]
- 6.Nagakawa Y, Sahara Y, Hosokawa Y, et al. Clinical Impact of Neoadjuvant Chemotherapy and Chemoradiotherapy in Borderline Resectable Pancreatic Cancer: Analysis of 884 Patients at Facilities Specializing in Pancreatic Surgery. Ann Surg Oncol. 2019;26(6):1629–1636. [DOI] [PubMed] [Google Scholar]
- 7.Cloyd JM, Chen HC, Wang X, et al. Chemotherapy Versus Chemoradiation as Preoperative Therapy for Resectable Pancreatic Ductal Adenocarcinoma: A Propensity Score Adjusted Analysis. Pancreas. 2019;48(2):216–222. [DOI] [PubMed] [Google Scholar]
- 8.Franko J, Hsu HW, Thirunavukarasu P, Frankova D, Goldman CD. Chemotherapy and radiation components of neoadjuvant treatment of pancreatic head adenocarcinoma: Impact on perioperative mortality and long-term survival. Eur J Surg Oncol. 2017;43(2):351–357. [DOI] [PubMed] [Google Scholar]
- 9.Versteijne E, Suker M, Groothuis K, et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J Clin Oncol. 2020:JCO1902274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Eijck CHJ, Versteijne E, Suker M, et al. Preoperative chemoradiotherapy to improve overall survival in pancreatic cancer: Long-term results of the multicenter randomized phase III PREOPANC trial. J Clin Oncol. 2021;39(15_suppl):4016–4016. [Google Scholar]
- 11.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. [DOI] [PubMed] [Google Scholar]
- 12.Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379(25):2395–2406. [DOI] [PubMed] [Google Scholar]
- 13.Janssen QP, Buettner S, Suker M, et al. Neoadjuvant FOLFIRINOX in Patients With Borderline Resectable Pancreatic Cancer: A Systematic Review and Patient-Level Meta-Analysis. JNCI: Journal of the National Cancer Institute. 2019;111(8):782–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garnier J, Ewald J, Marchese U, et al. Borderline or locally advanced pancreatic adenocarcinoma: A single center experience on the FOLFIRINOX induction regimen. Eur J Surg Oncol. 2020;46(8):1510–1515. [DOI] [PubMed] [Google Scholar]
- 15.Auclin E, Marthey L, Abdallah R, et al. Role of FOLFIRINOX and chemoradiotherapy in locally advanced and borderline resectable pancreatic adenocarcinoma: update of the AGEO cohort. Br J Cancer. 2021;124(12):1941–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maggino L, Malleo G, Marchegiani G, et al. Outcomes of Primary Chemotherapy for Borderline Resectable and Locally Advanced Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2019;154(10):932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao XI, Wang X, Speicher PJ, et al. Reporting and Guidelines in Propensity Score Analysis: A Systematic Review of Cancer and Cancer Surgical Studies. J Natl Cancer Inst. 2017;109(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kakar S, Pawlik TM, Allen PJ. AJCC Cancer Staging Manual (ed 8th Edition). New York, NY: Springer-Verlag; 2016. [Google Scholar]
- 19.The Royal College of Pathologists. Dataset for the histopathological reporting of carcinomas of the pancreas, ampulla of Vater and common bile duct.; 2017.
- 20.Lee SM, Katz MH, Liu L, et al. Validation of a Proposed Tumor Regression Grading Scheme for Pancreatic Ductal Adenocarcinoma After Neoadjuvant Therapy as a Prognostic Indicator for Survival. Am J Surg Pathol. 2016;40(12):1653–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen J Statistical power analysis for the behavioral sciences [chapter 2]. Toronto: Academic Press, Inc.; 1977. [Google Scholar]
- 22.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009, Nov;28(25):3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz MHG, Ou FS, Herman JM, et al. Alliance for clinical trials in oncology (ALLIANCE) trial A021501: preoperative extended chemotherapy vs. chemotherapy plus hypofractionated radiation therapy for borderline resectable adenocarcinoma of the head of the pancreas. BMC Cancer. 2017;17(1):505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katz MHG, Shi Q, Meyers JP, et al. Alliance A021501: Preoperative mFOLFIRINOX or mFOLFIRINOX plus hypofractionated radiation therapy (RT) for borderline resectable (BR) adenocarcinoma of the pancreas. J Clin Oncol. 2021;39(3_suppl):377–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong J, Patel K, Switchenko J, et al. Outcomes for patients with locally advanced pancreatic adenocarcinoma treated with stereotactic body radiation therapy versus conventionally fractionated radiation. Cancer. 2017;123(18):3486–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tchelebi LT, Lehrer EJ, Trifiletti DM, et al. Conventionally fractionated radiation therapy versus stereotactic body radiation therapy for locally advanced pancreatic cancer (CRiSP): An international systematic review and meta-analysis. Cancer. 2020;126(10):2120–2131. [DOI] [PubMed] [Google Scholar]
- 27.de Geus SWL, Eskander MF, Kasumova GG, et al. Stereotactic body radiotherapy for unresected pancreatic cancer: A nationwide review. Cancer. 2017;123(21):4158–4167. [DOI] [PubMed] [Google Scholar]
- 28.Janssen QP, van Dam JL, Kivits I, Besselink MG, van Eijck CHJ, et al. The added value of radiotherapy following neoadjuvant FOLFIRINOX for resectable and borderline resectable pancreatic cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2021(13):8297–8308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammel P, Huguet F, van Laethem JL, et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA. 2016;315(17):1844–1853. [DOI] [PubMed] [Google Scholar]
- 30.Pietrasz D, Turrini O, Vendrely V, et al. How Does Chemoradiotherapy Following Induction FOLFIRINOX Improve the Results in Resected Borderline or Locally Advanced Pancreatic Adenocarcinoma? An AGEO-FRENCH Multicentric Cohort. Ann Surg Oncol. 2019;26(1):109–117. [DOI] [PubMed] [Google Scholar]
- 31.Xiang M, Heestand GM, Chang DT, Pollom EL. Neoadjuvant treatment strategies for resectable pancreas cancer: A propensity-matched analysis of the National Cancer Database. Radiother Oncol. 2020;143:101–107. [DOI] [PubMed] [Google Scholar]
- 32.Hue JJ, Dorth J, Sugumar K, et al. Neoadjuvant Radiotherapy is Associated With Improved Pathologic Outcomes and Survival in Resected Stage II-III Pancreatic Adenocarcinoma Treated With Multiagent Neoadjuvant Chemotherapy in the Modern Era. Am Surg. 2021:31348211038581. [DOI] [PubMed] [Google Scholar]
- 33.Cloyd JM, Ejaz A, Shen C, et al. Pathologic complete response following neoadjuvant therapy for pancreatic ductal adenocarcinoma: defining the incidence, predictors, and outcomes. HPB (Oxford). 2020;22(11):1569–1576. [DOI] [PubMed] [Google Scholar]
- 34.Verbeke CS. Resection margins in pancreatic cancer. Surg Clin North Am. 2013;93(3):647–662. [DOI] [PubMed] [Google Scholar]
- 35.Sobin LH, Gospodarowicz MK, Wittekind C et al. TNM classification of malignant tumours (seventh ed.): Wiley Blackwell, Oxford; 2011. [Google Scholar]
- 36.Soer EC, Verbeke CS. Pathology reporting of margin status in locally advanced pancreatic cancer: challenges and uncertainties. J Gastrointest Oncol. 2021;12(5):2512–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Dongen JC, Suker M, Versteijne E, et al. Surgical Complications in a Multicenter Randomized Trial Comparing Preoperative Chemoradiotherapy and Immediate Surgery in Patients With Resectable and Borderline Resectable Pancreatic Cancer (PREOPANC Trial). Ann Surg. 2020. [DOI] [PubMed] [Google Scholar]
- 38.van Dongen JC, Wismans LV, Suurmeijer JA, et al. The effect of preoperative chemotherapy and chemoradiotherapy on pancreatic fistula and other surgical complications after pancreatic resection: a systematic review and meta-analysis of comparative studies. HPB (Oxford). 2021;23(9):1321–1331. [DOI] [PubMed] [Google Scholar]
- 39.Gao S, Zhu X, Shi X, et al. Comparisons of different neoadjuvant chemotherapy regimens with or without stereotactic body radiation therapy for borderline resectable pancreatic cancer: study protocol of a prospective, randomized phase II trial (BRPCNCC-1). Radiation Oncology. 2019;14(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janssen QP, van Dam JL, Bonsing BA, et al. Total neoadjuvant FOLFIRINOX versus neoadjuvant gemcitabine-based chemoradiotherapy and adjuvant gemcitabine for resectable and borderline resectable pancreatic cancer (PREOPANC-2 trial): study protocol for a nationwide multicenter randomized controlled trial. BMC Cancer. 2021;21(1):300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol. 2013;31(23):2963–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


